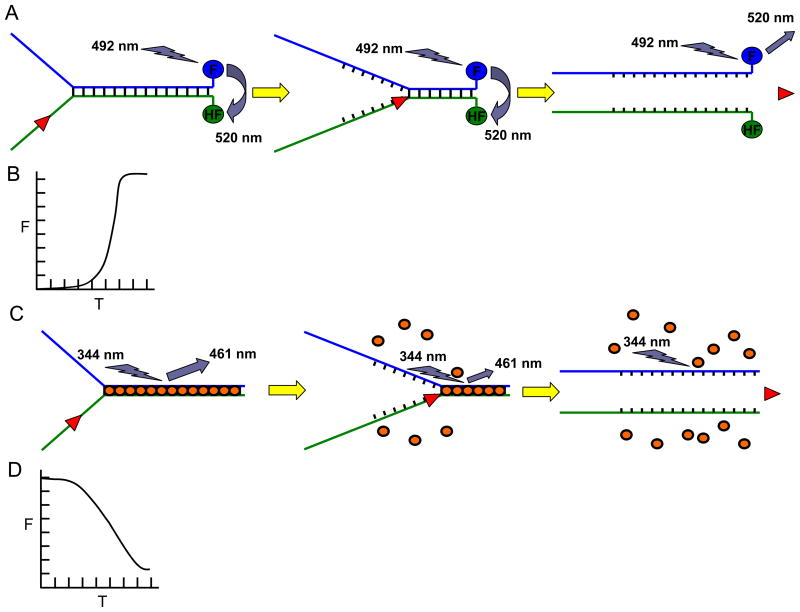
Fig. 1. Fluorescence-based helicase assays.
Panel A, Schematic of the FRET-based helicase assay to measure helicase activity. Fluorescein (F) is covalently attached to the 3′ blunt end and hexachlorofluorescein (HF) is attached to the 5′ blunt end of the forked duplex DNA substrate. When in close proximity, HF quenches the signal emitted from F upon excitation. Upon unwinding, the emission from F upon excitation is free to be detected because the HF is no longer in close proximity with F. Note that all-or-none unwinding of the DNA substrate is measured using the FRET-based assay. Panel B, Kinetics of fluorescence increase (as a measure of helicase activity on the forked duplex substrate) according to time as measured by the FRET-based helicase assay. Panel C, Schematic of the dye displacement assay to measure helicase activity. The dye molecules are pre-bound to the duplex DNA substrate (gold ovals), causing them to fluoresce. As the dsDNA is unwound, the dye molecules are released which is measured by a decrease in the amount of fluorescence. Note that partial unwinding by a helicase can be measured using the dye displacement assay. Panel D, Kinetics of fluorescence (F) decrease (as a measure of helicase activity on the forked duplex substrate) according to time (T) as measured by the dye-displacement assay.