Fig. 1.
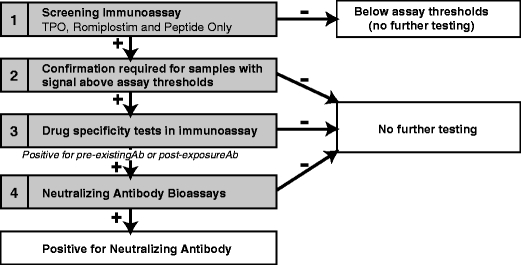
Process for assessment of immunogenicity in the romiplostim clinical trial program. The strategy for immunogenicity assessment involved a screening step where the serum samples were assessed for their ability to bind to TPO, romiplostim, and peptide component of romiplostim. If the sample showed binding above the validated assay threshold, it was further confirmed in a specificity test. Based on the reactivity observed, excess of the relevant TPO or romiplostim was added to the reactive sample and assessed for neutralization of the reactive response. If the sample exhibited more than 50% depletion of signal in drug specificity analysis, the sample was then confirmed for its ability to neutralize romiplostim or TPO in a biological functional assay
