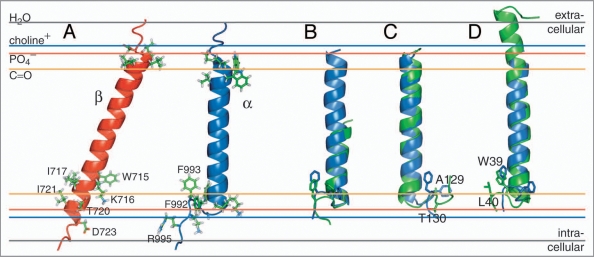
Figure 2.
NMR structures of the individual integrin αIIb and β3 transmembrane segments. (A) Structures of the monomeric αIIb and β3 TM segments (PDB entries 2k1a and 2rmz, respectively) and their estimated membrane embedding.12,13,18,19 Selected side chains are shown in ball-and-stick representation. (B) An αIIb-homologous GFF structural motif is found in glycogen phosphorylase b (GPb; PDB entry 1a8i;22 residues 748–750). The αIIb TM segment and pertinent GPb helix are shown in blue and green, respectively. (C) For αIIb (blue), structural homology was found for a TM helix of cytochrome c oxidase (PDB entry 1xme, chain A), shown in green. The side chains of αIIb(F992–F993) and 1xme-A (A129-T130) are shown in ball-and-stick representation. F992 and A129 are structurally homologous. (D) The β peptide from the light-harvesting protein B-800/850 (homologous PDB entries 2fkw, chain S; 1nkz, chain F; and 1kzu, chain B also exhibits homology to αIIb. Shown is the structural alignment of integrin αIIb(P965–R995), in blue, with chain F of 1nkz, in green. The side chains of αIIb(F992–F993) and 1nkz-F(W39–L40) are shown in ball-and-stick representation. F993 and W39 are structurally homologous. The experimentally determined average positions of the lipid carbonyl, phosphate and choline groups (16, 20 and 22 Å from the center of the membrane) are depicted for 1,2-Dioleoyl-sn-Glycero-3-Phosphocholine.54 The bulk water phase is assumed to be fully established at 28 Å.55