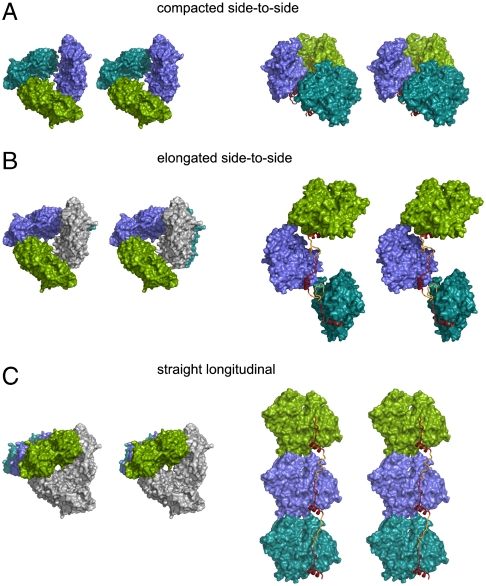
Fig. 3.
The two main architectures of packing in the SpirBCD/AP-actin crystal. Actin molecules are shown as surface plots, WH2 domains as ribbon plots in red/yellow. “Missing” linkers are depicted as ribbons and colored in yellow. (Left) Views along the axis of actin-nucleation seed. (Right) Side views ca. perpendicular to the long axis of the actin-nucleation seed. A and B show the side-to-side architectures. (A) The primary nucleus (colored green, blue, and cyan) is built by the Spire/AP-actin complex inside one asymmetric unit (compacted side-to-side). (B) The primary nucleus is built by the Spire/AP-actin complex, which belongs to two neighboring asymmetric units. Actin in gray belongs to the asymmetric unit that contains the two other actins in green and blue. (C) shows the straight-longitudinal architecture of packing in the SpirBCD/AP-actin crystal. In this model one molecule of actin (i.e., the molecule colored in green) and its two symmetry related ones (actins in blue and cyan) constitute an actin-nucleation seed. Actins in gray belong to the asymmetric unit that contains the actin shown in green.