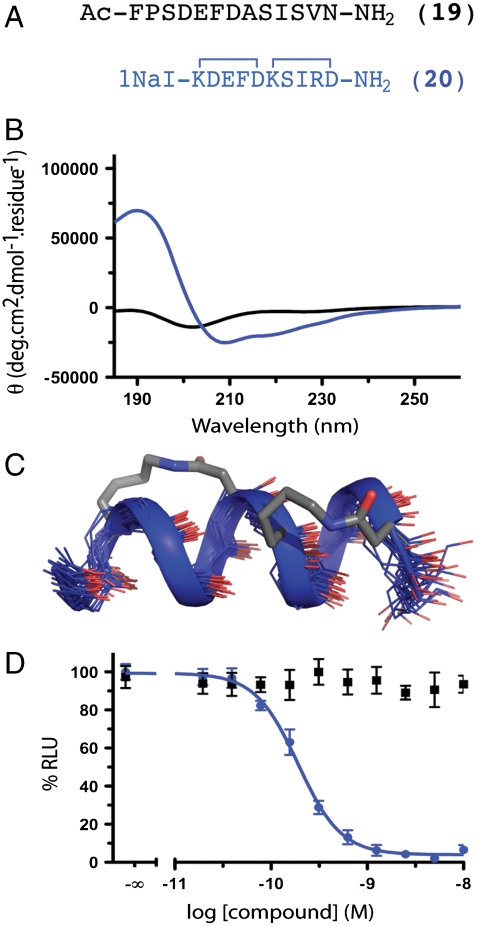
Fig. 3.
RSV fusion peptides. (A) RSV F glycoprotein HR-C (residues F483–V495) mimicked by 20 vs.unconstrained 19. (B and C) The unconstrained sequence 19 had no structure whereas 20 showed an α-helical CD spectrum and NMR structure. The CD spectra of the linear and helix-constrained RSV peptide suggested that the constrained analogue had significantly more helicity. The structure was confirmed through 1H NMR. (D) Helix-constrained 20 inhibited recombinant RSV F mediated fusion in a cell-to-cell assay at picomolar concentrations (190 pM, pIC50 ≤ 9.72 ± 0.03; blue) whereas unconstrained 19 showed no inhibition < 1 μM (black). Error bars represented ± S.E.M with n≥3.