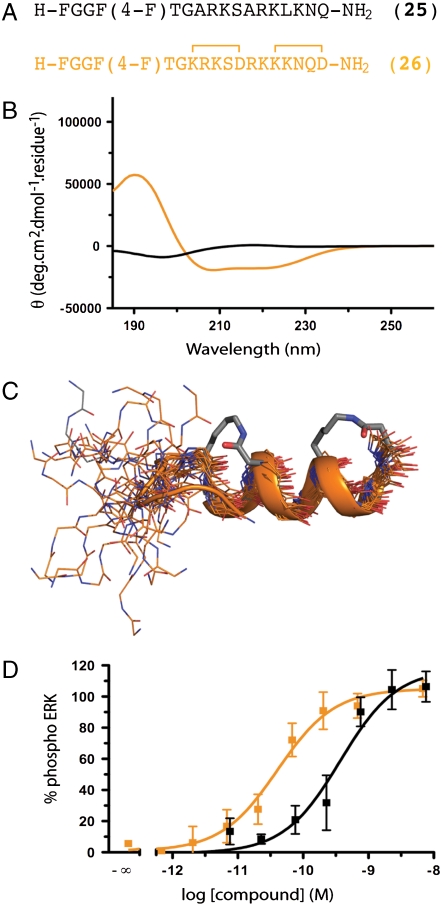
Fig. 6.
GPCR-ligand nociceptin. (A) Nociceptin residues 1–17 (25) is a superagonist of ORL-1. (B) The native nociceptin(1-17) sequence had no structure in water but a recent structure in 10 mM SDS suggests R8-Q17 has α-helical propensity. (C) Cyclic mimetic 26 showed α-helicity between R8-Q17 whereas N-terminal heptapeptide had some turn-like structure. (D) Helix-constrained nociceptin analogue 26 was a 9-fold more potent ORL-1 agonist (EC50 40 pM, pEC50 ≤ 10.39 ± 0.14; orange), than unconstrained 25 (360 pM, pEC50 ≤ 9.43 ± 0.17; black) as assessed by ERK phosphorylation in mouse neuroblastoma cells Neuro-2a. Error bars represented ± S.E.M with n≥3. F(4-F) = parafluorophenylalanine.