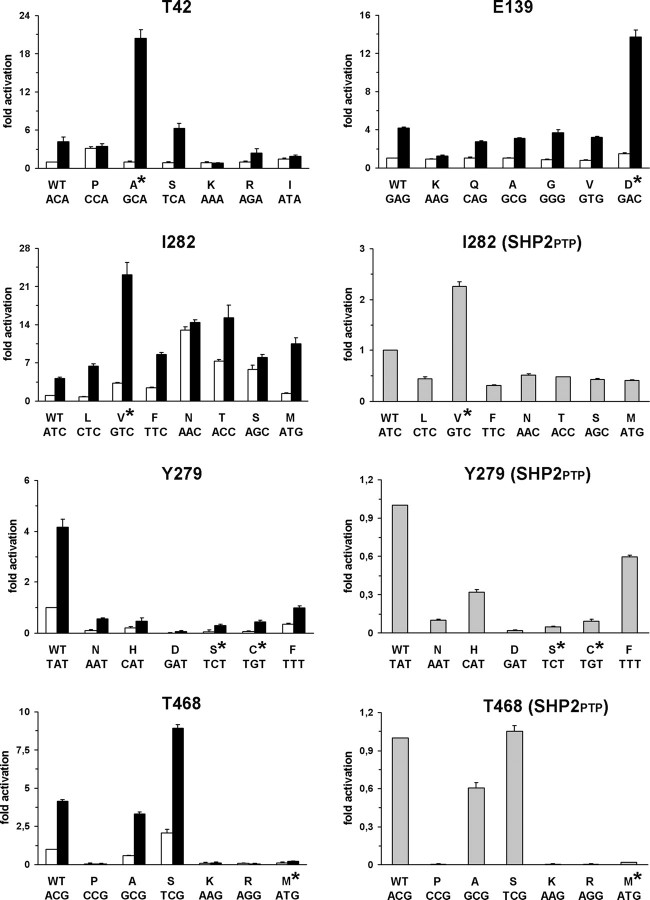
Figure 1.
Biochemical characterization of SHP2 mutants. In vitro phosphatase assay of wild-type SHP2 and all possible mutants arising from a single-base change at codons 42, 139, 282, 279 and 468. Catalytic activity was measured as pmoles of phosphate released using pNPP as substrate, basally (white bars) and following stimulation with the PTPNS1 BTAM peptide (black bars). Activities of recombinant wild-type SHP2PTP encoding for the isolated PTP domain and mutants at positions 282, 279 and 468 are also reported (gray bars). Values are expressed as mean ± standard deviation of at least three independent experiments and are normalized to the basal activity of the wild-type enzyme.