Abstract
Accumulating evidence suggests that autophagy can be selective in the clearance of organelles in yeast and in mammalian cells. We have observed that the sequestration of mitochondria by autophagosomes was defective in reticulocytes in the absence of Nix. Nixis required for the dissipation of mitochondrial membrane potential (ΔΨm) during erythroid maturation. Moreover, pharmacological agents that induce the loss of ΔΨm can restore the sequestration of mitochondria by autophagosomes and promote mitochondrial clearance in Nix−/− erythroid cells. Our data suggest that mitochondrial depolarization induces recognition and sequestration of mitochondria by autophagosomes. Elucidating the mechanisms underlying selective mitochondrial autophagy not only will help us to understand the mechanisms for erythroid maturation, but also may provide insights into mitochondrial quality control by autophagy in the protection against aging, cancer, and neurodegenerative diseases.
Keywords: Nix, autophagy, erythroid maturation, Bcl-2, BH3, mitochondrial membrane potential, mitochondrial quality control
Autophagy is emerging as an important mechanism for regulating the development and functions of a variety of cell types1, 2. Autophagy plays an important role in the degradation of cellular organelles, RNA and proteins in multiple cellular processes. The inclusion of cytoplasmic components by autophagosomes for macroautophagy has been considered to be largely a non-specific event. However, accumulating evidence suggests that mitochondrial autophagy can be selectively regulated in the yeast3, 4. To understand the functions of autophagy in multiple cellular processes, it will be important to define the molecular events involved in the regulation of selective organelle degradation by autophagy.
Bcl-2 family proteins play an essential role in regulating mitochondrion-dependent apoptosis5–7. Their roles in regulating autophagy have also received increasing attentions2. Binding of Bcl-2 or Bcl-xL to Beclin 1 has been shown to inhibit autophagy, and BH3-only members of the Bcl-2 family may bind to and sequester Bcl-2/Bcl-xL, thereby releasing Beclin 1 from such inhibition to induce autophagy8–10. An atypical BH3-only protein, Bnip3, has been shown to mediate hypoxia-induced autophagy11. Here we will discuss the roles for Nix, a homolog of Bnip3, in the regulation of mitochondrial autophagy.
Defective clearance of mitochondria in reticulocytes and the development of hemolytic anemia in the absence of Nix
While studying the regulation of apoptosis in the immune system, we observed that several BH3-only proteins, including Bim and Nix, can be induced by stimulation via toll-like receptors in dendritic cells12. Because of the potential roles for Nix in mediating apoptosis siganling13, 14, we generated Nix-deficient mice to investigate whether Nix might regulate apoptosis in the immune system. However, we did not find significant changes in dendritic cells or other immune cell types in Nix−/− mice. Unexpectedly, we observed prominent defects in the erythroid compartment of Nix−/− mice. During terminal differentiation, erythroid cells undergo enucleation to become reticulocytes, followed by coordinated removal of organelles to form mature red blood cells (RBCs)15, 16. Nix−/− mice contained increased reticulocytes and reduced mature RBCs that are characteristic of hemolytic anemia17. The increases in the production of erythropoietin suggests a feedback mechanism for reduced mature RBCs in stimulating erythropoiesis in Nix−/− mice17, 18. Another study suggests that the resistance of Nix−/− erythroblasts to cell death contributes to increased erythroid precursors in Nix−/− mice19.
Nix−/− RBCs showed abnormal retention of mitochondria17, 18. The loading and unloading of oxygen can induce oxidative stress in RBCs20. Because the mitochondrion is a major site for the generation of reactive oxygen species (ROS) and can also function as an apoptotic machinery21, the retention of mitochondria is potentially harmful to RBCs. We observed that Nix−/− RBCs produced more ROS, displayed more caspase activation in vitro and underwent faster turnover in vivo18. This supports the conclusion that RBCs with abnormal retention of mitochondria are prone to oxidative stress-induced cell death.
Autophagy has been implicated in terminal erythroid differentiation22, 23. Interestingly, Nix deficiency does not affect the formation of autophagosomes in reticulocytes. Instead, we found that mitochondria were clustered outside of autophagosomes in Nix−/− reticulocytes17, 18. This indicates that Nix is required for the sequestration of mitochondria by autophagosomes17, 18. While our revised manuscript was under consideration in Nature, another group using virally transformed Nix−/− erythroid cells also reported the observation of defective inclusion of mitochondria by autophagosomes24. These studies suggest an essential role for Nix in the regulation of selective mitochondrial autophagy during erythroid maturation. In contrast to defective mitochondrial clearance, various aspects of erythroid maturation, including the removal of nucleus and ribosomes, as well as CD71 downregulation, can proceed despite a defect in mitochondrial clearance in Nix−/− RBCs17, 18. Therefore, defective mitochondrial clearance in the absence of Nix is not due to a general block in autophagy or erythroid maturation.
Nix-dependent loss of mitochondrial membrane potential in the promotion of mitochondrial autophagy
Our studies further demonstrated that Nix−/− reticulocytes were defective in the loss of mitochondrial membrane potential (ΔΨm) during maturation18. Treatment with FCCP, an uncoupling agent that dissipates the proton gradient across mitochondrial inner membrane, dissipated ΔΨm and restored the sequestration of mitochondria by autophagosomes in Nix−/− RBCs17, 18. Treatment with ABT-737, a BH3 mimetic, also had similar effects18. These data indicate that Nix-dependent loss of ΔΨm promotes mitochondrial autophagy17, 18. We have also observed that promoting mitochondrial depolarization helps to correct defective mitochondrial autophagy in Nix−/− RBCs in vivo (our unpublished data). These studies may provide valuable insights into the development of therapeutic approaches in treating various diseases involving defective mitochondrial autophagy.
In contrast to the effects of FCCP and ABT-737, an F0F1-ATPase inhibitor, oligomycin25, did not induce the loss of ΔΨm or promote mitochondrial clearance in Nix−/− RBCs18, suggesting that inhibiting ATP synthesis without disrupting ΔΨm is not sufficient to induce mitophagy. Treatment with ROS scavengers did not inhibit the loss of mitochondria in FCCP-treated Nix−/− RBCs18, indicating that ROS is not responsible for FCCP-mediated rescue of mitochondrial autophagy in Nix−/− RBCs.
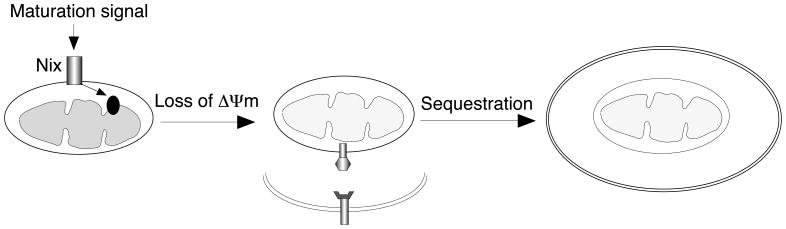
How depolarized mitochondria might be selectively sequestered by autophagosomes is unclear. Consistent with our findings that loss of ΔΨm is important for selective mitochondrial autophagy, a recent study shows that mitochondria with reduced ΔΨm and decreased expression of a mitochondrial fusion protein, OPA1, are preferentially targeted for autophagy26. Whether the loss of ΔΨm affects mitochondrial dynamics to regulate mitochondrial autophagy remains to be determined. We propose that the loss of the electrochemical gradient across the inner mitochondrial membranes results in differential display of molecules on the outer membranes of mitochondria, facilitating the interactions with autophagosomal proteins to promote mitochondrial autophagy (Fig. 1). Further identification of these potential molecules will help us to understand mechanisms underlying selective mitochondrial autophagy. Ulk1, a mammlian homolog of yeast Atg1, has recently been implicated in regulating mitophagy in RBCs27. It will be interesting to test whether Ulk1 is required for autophagosomes to target depolarized mitochondria.
Figure 1.
A model for Nix-dependent mitochondrial autophagy. During terminal erythroid differentiation, maturation signals may activate Nix to signal into mitochondria to dissipate ΔΨm. The loss of the electrochemical gradient across the inner mitochondrial membrane may induce the differential display of molecules on the outer mitochondrial membrane. The interactions of these molecules on mitochondria with autophagosomal proteins may lead to selective sequestration of the depolarized mitochondria into autophagosomes.
The signaling pathway downstream of Nix in inducing the loss of ΔΨm is currently under study. As a mitochondrial outer membrane protein, Nix contains a short c-terminus tail in the mitochondrial intermembrane space. This intra-mitochondria tail may interact with other proteins in mitochondria to disrupt the electrochemical gradient across the inner membrane (Fig. 1). It is interesting to note that Aup1p is a mitochondrial phosphatase that promotes mitochondrial autophagy in yeast4. It remains to be determined whether the mammalian homolog of this mitochondrial phosphatase might mediate the Nix-dependent loss of ΔΨm to promote mitochondrial autophagy in erythroid cells.
The upregulation of Nix during terminal erythroid differentiation28 may be important for the expression of sufficient Nix to promote mitochondrial autophagy. Nix deficiency does not affect mitochondria mass in lymphocytes which do not lose mitochondria developmentally18. Why a defect in Nix causes significant phenotype in erythroid cells, but not in other cell types is unknown. It has been suggested that each BH3-only protein in the Bcl-2 family serves as specific sensors for different apoptosis stimuli29. Likewise, the roles of Nix in mediating mitophagy may also be specific in erythroid cells. We observed that deficiency in Bim but not Nix affects T cell development, whereas deficiency in Nix but not Bim impedes erythroid maturation18. We propose that Nix is a specific sensor for the maturation signals in RBCs undergoing terminal differentiation (Fig. 1), whereas Bim does not respond to such maturation signals in RBCs. After triggering by the maturation signals, Nix potentially interacts with intra-mitochondrial molecules to induce the loss of mitochondrial membrane potential and promotes mitochondrial autophagy. Characterizing the maturation signal will provide insights into the mechanism for the activation of Nix during erythroid differentiation.
In summary, our study suggests that Nix-dependent loss of ΔΨm leads to specific recognition and sequestration of mitochondria by autophagosomes during erythroid maturation (Fig. 1). Successful clearance of mitochondria may prevent mitochondrion-dependent caspase activation, reduce oxidative stress and sustain the survival of erythrocytes. However, several questions remain to be answered. How does Nix sense the maturation signals in differentiating erythroid cells? What are the molecules downstream of Nix to mediate the loss of ΔΨm? How do autophagosomes recognize depolarized mitochondria for selective sequestration? Mitochondrial autophagy is emerging as an important mechanism for mitochondrial quality control in the protection against aging, cancer, diabetes, and neurodegenerative diseases30. Studying molecular mechanisms underlying mitochondrial autophagy in erythroid cells may shed light on the regulation of autophagy in the development of other cell types, as well as in various disease processes involving defective mitochondrial autophagy.
Acknowledgments
This work was supported by grants from the American Hematology Society, American Heart Association and the NIH.
Footnotes
Addendum to: Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, Wang J. Essential role for Nix in autophagic maturation of erythroid cells. Nature 2008; 454:232-5.
References
- 1.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–77. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 2.Levine B, Sinha S, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–6. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kissova I, Deffieu M, Manon S, Camougrand N. Uth1p is involved in the autophagic degradation of mitochondria. J Biol Chem. 2004;279:39068–74. doi: 10.1074/jbc.M406960200. [DOI] [PubMed] [Google Scholar]
- 4.Tal R, Winter G, Ecker N, Klionsky DJ, Abeliovich H. Aup1p, a yeast mitochondrial protein phosphatase homolog, is required for efficient stationary phase mitophagy and cell survival. J Biol Chem. 2007;282:5617–24. doi: 10.1074/jbc.M605940200. [DOI] [PubMed] [Google Scholar]
- 5.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 6.Rathmell JC, Thompson CB. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell. 2002;109 (Suppl):S97–107. doi: 10.1016/s0092-8674(02)00704-3. [DOI] [PubMed] [Google Scholar]
- 7.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–6. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 8.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, Hickman JA, Geneste O, Kroemer G. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. Embo J. 2007;26:2527–39. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Molecular cell. 2008;30:678–88. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tracy K, Dibling BC, Spike BT, Knabb JR, Schumacker P, Macleod KF. BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Molecular and cellular biology. 2007;27:6229–42. doi: 10.1128/MCB.02246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen M, Wang YH, Wang Y, Huang L, Sandoval H, Liu YJ, Wang J. Dendritic cell apoptosis in the maintenance of immune tolerance. Science. 2006;311:1160–4. doi: 10.1126/science.1122545. [DOI] [PubMed] [Google Scholar]
- 13.Chen G, Cizeau J, Vande Velde C, Park JH, Bozek G, Bolton J, Shi L, Dubik D, Greenberg A. Nix and Nip3 form a subfamily of pro-apoptotic mitochondrial proteins. J Biol Chem. 1999;274:7–10. doi: 10.1074/jbc.274.1.7. [DOI] [PubMed] [Google Scholar]
- 14.Yussman MG, Toyokawa T, Odley A, Lynch RA, Wu G, Colbert MC, Aronow BJ, Lorenz JN, Dorn GW., 2nd Mitochondrial death protein Nix is induced in cardiac hypertrophy and triggers apoptotic cardiomyopathy. Nat Med. 2002;8:725–30. doi: 10.1038/nm719. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida H, Kawane K, Koike M, Mori Y, Uchiyama Y, Nagata S. Phosphatidylserine-dependent engulfment by macrophages of nuclei from erythroid precursor cells. Nature. 2005;437:754–8. doi: 10.1038/nature03964. [DOI] [PubMed] [Google Scholar]
- 16.Koury MJ, Koury ST, Kopsombut P, Bondurant MC. In vitro maturation of nascent reticulocytes to erythrocytes. Blood. 2005;105:2168–74. doi: 10.1182/blood-2004-02-0616. [DOI] [PubMed] [Google Scholar]
- 17.Chen M, Prchal JT, Hector S, Thiagarajan P, Dasgupta SK, Schumacher A, Wang J. Essential role of pro-apoptotic mechanisms for production of normal erythrocytes and prevention of hemolysis. Blood. 2007;110:132A. [Google Scholar]
- 18.Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, Wang J. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454:232–5. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diwan A, Koesters AG, Odley AM, Pushkaran S, Baines CP, Spike BT, Daria D, Jegga AG, Geiger H, Aronow BJ, Molkentin JD, Macleod KF, Kalfa TA, Dorn GW., 2nd Unrestrained erythroblast development in Nix−/− mice reveals a mechanism for apoptotic modulation of erythropoiesis. Proc Natl Acad Sci U S A. 2007;104:6794–9. doi: 10.1073/pnas.0610666104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sivilotti ML. Oxidant stress and haemolysis of the human erythrocyte. Toxicol Rev. 2004;23:169–88. doi: 10.2165/00139709-200423030-00004. [DOI] [PubMed] [Google Scholar]
- 21.Raha S, Robinson BH. Mitochondria, oxygen free radicals, and apoptosis. Am J Med Genet. 2001;106:62–70. doi: 10.1002/ajmg.1398. [DOI] [PubMed] [Google Scholar]
- 22.Kent G, Minick OT, Volini FI, Orfei E. Autophagic vacuoles in human red cells. Am J Pathol. 1966;48:831–57. [PMC free article] [PubMed] [Google Scholar]
- 23.Heynen MJ, Tricot G, Verwilghen RL. Autophagy of mitochondria in rat bone marrow erythroid cells. Relation to nuclear extrusion. Cell Tissue Res. 1985;239:235–9. doi: 10.1007/BF00214924. [DOI] [PubMed] [Google Scholar]
- 24.Schweers RL, Zhang J, Randall MS, Loyd MR, Li W, Dorsey FC, Kundu M, Opferman JT, Cleveland JL, Miller JL, Ney PA. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc Natl Acad Sci U S A. 2007;104:19500–5. doi: 10.1073/pnas.0708818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rego AC, Vesce S, Nicholls DG. The mechanism of mitochondrial membrane potential retention following release of cytochrome c in apoptotic GT1-7 neural cells. Cell Death Differ. 2001;8:995–1003. doi: 10.1038/sj.cdd.4400916. [DOI] [PubMed] [Google Scholar]
- 26.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. Embo J. 2008;27:433–46. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kundu M, Lindsten T, Yang CY, Wu J, Zhao F, Zhang J, Selak MA, Ney PA, Thompson CB. Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood. 2008 doi: 10.1182/blood-2008-02-137398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aerbajinai W, Giattina M, Lee YT, Raffeld M, Miller JL. The proapoptotic factor Nix is coexpressed with Bcl-xL during terminal erythroid differentiation. Blood. 2003;102:712–7. doi: 10.1182/blood-2002-11-3324. [DOI] [PubMed] [Google Scholar]
- 29.Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- 30.Tatsuta T, Langer T. Quality control of mitochondria: protection against neurodegeneration and ageing. Embo J. 2008;27:306–14. doi: 10.1038/sj.emboj.7601972. [DOI] [PMC free article] [PubMed] [Google Scholar]