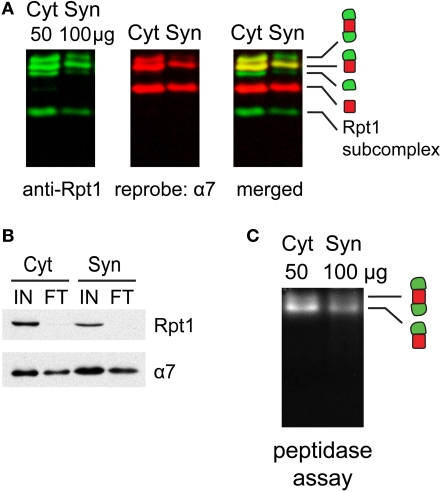
Figure 3.
(A) Proteasome integrity after subcellular fractionation. Cytosolic (Cyt) and synaptic (Syn) extracts prepared from rat cortices were resolved by 2–5% gradient native gel, immunoblotted against 19S subunit Rpt1, and reprobed for 20S subunit α7. Singly-, doubly-capped 26S, 20S proteasomes and free 19S were detected in both fractions. (B) Efficacy of 26S proteasome isolation by UBL affinity chromatography. Cytosolic (Cyt) and synaptic (Syn) extracts were incubated with GST-UBL and glutathione sepharose. The input (IN) and flow-through (FT) materials were analyzed by SDS-PAGE and immunoblotted against Rpt1 and α7. The depletion of Rpt1 from the flow-through indicated that most 26S proteasomes and 19S particles remained intact during subcellular fractionation and were captured with high efficiency. The high remaining level of α7 subunit in the flow-through is consistent with the abundance of free 20S particles detected in (A). (C) Cytosolic and synaptosomal extracts contain active 26S proteasomes. Cytosolic (Cyt) and synaptic (Syn) extracts isolated from rat cortices were resolved by 2–5% gradient native gel, followed by incubation with Suc-LLVY-AMC, a fluorogenic proteasome substrate. Active 26S proteasomes appear as fluorescent bands, corresponding to doubly- and singly-capped 26S proteasomes.