Figure 5.
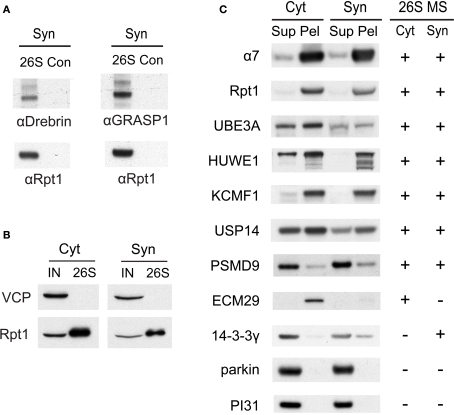
(A) Verification of drebrin and GRASP-1 as interactors of synaptic proteasomes. Both proteins were initially detected in synaptic proteasomes by mass spectrometry, based on a single matching peptide (Table 2). These specific interactions are confirmed by SDS-PAGE and Western blotting. Synaptosome (Syn)-derived proteasomes (26S) and control (Con) purifications using only GST are probed with specific antibodies against drebrin, GRASP-1, and 19S subunit Rpt1. (B) The VCP complex does not interact appreciably with GST-UBL. 26S proteasomes from the cytosolic (Cyt) and synaptic (Syn) extracts were purified by the GST-UBL/His-UIM method. The input (IN) material and purified 26S proteasomes were immunoblotted against VCP and Rpt1. The amount of VCP captured by the GST-UBL matrix was undetectable by Western blot in three independent experiments. (C) Verification of proteasome-interacting proteins by co-sedimentation. Cytosolic (Cyt) and synaptosomal (Syn) extracts were subjected to centrifugation at 100,000 × g for 6 h. The supernatant (Sup) and pellet (Pel) materials were loaded at 1:1 ratio and resolved by SDS-PAGE. The sedimentation of proteasomes was confirmed by the high levels of 20S subunit α7 and 19S subunit Rpt1 in the pellet fraction. Proteins identified by mass spectrometry in 26S proteasomes (26S MS) from the cytosol and the synaptosome are indicated by the positive sign. Agreement between mass spectrometry data and co-sedimentation data was observed for all seven 26S-interacting proteins (UBE3A, HUWE1, KCMF1, USP14, PSMD9, ECM29, 14-3-3γ) probed by specific antibodies (see Materials and Methods). Parkin and PI31 did not show appreciable association with brain proteasomes. Results shown here are representative of three experiments.