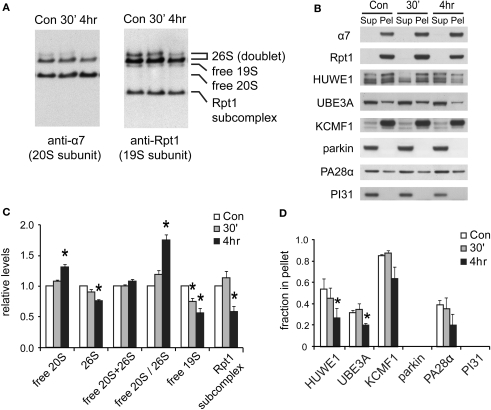
Figure 7.
Changes in proteasome complexes after NMDA exposure. (A) The disassembly of 26S proteasomes. Neuronal lysates collected at 30’ and 4 h post-NMDA (20 μM, 3 min) were resolved by 2–5% gradient native gel and immunoblotted against 20S subunit α7 and 19S subunit Rpt1. (B) Changes in proteasome-associated proteins. Lysates from (A) were subjected to ultracentrifugation to sediment proteasomes. Equal amounts of supernatant (Sup) and pellet (Pel) materials were analyzed by SDS-PAGE. The sedimentation property of proteasome-interacting proteins (HUWE1, UBE3A, KCMF1, PA28α) was examined by Western blotting. Parkin and PI31 were not detected in the pellet. (C) Quantification of changes in proteasome distributions in (A) by densitometry (n = 6, mean ± SEM, *p < 0.05 by paired t-test). (D) The graph represents the sedimentation data in (B). The protein level in the pellet is divided by the total level (supernatant + pellet), and the results are plotted (n = 4, *p < 0.05 by paired t-test).