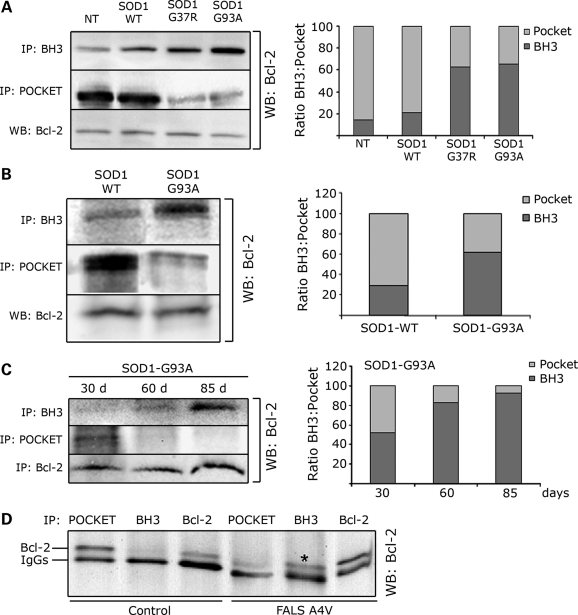
Figure 4.
MutSOD1s induce a conformational change in Bcl-2 leading to exposure of the toxic BH3 domain in cells, mouse and human spinal cords. (A) SH-SY5Y cells, which express endogenous Bcl-2, were transfected with SOD1 (WT, G37R or G93A) and Bcl-2 conformation assessed by immunoprecipitation with the α-Bcl-2/BH3 and α-Bcl-2/pocket antibody, respectively. Immunoprecipitated proteins were analyzed by WB with anti-Bcl-2 antibody against the N-terminal domain (left panel). Total amount of Bcl-2 was assessed by WB (left panel, bottom lane). In the presence of mutSOD1s (G37R and G93A), there is an increased exposure of the toxic BH3 domain paralleled by a decrease in the pocket region. The plot in the right panel shows the ratio BH3/pocket as analyzed by densitometric analysis of the immunoprecipitated Bcl-2 with the Quantity One software. (B) The α-Bcl-2/BH3 and α-Bcl-2/pocket antibodies were used to immunoprecipitate Bcl-2 from spinal cord homogenates of 130-day-old transgenic mice expressing either SOD1-WT or SOD1-G93A. In SOD1-G93A mice there is an increased exposure of the toxic BH3 domain of Bcl-2 compared with age-matched transgenic SOD1-WT mice, and this is paralleled by a decreased exposure of the pocket. (C) Conformational changes in Bcl-2 were then assessed over time in the SOD1-G93A mice. Exposure of the toxic BH3 domain appears prior to and peaks at disease onset (left panel). Densitometry analysis of the ratio BH3/pocket is shown in the plot on the right. (D) Immunoprecipitation of post-mortem human SOD1-A4V spinal cord shows increased exposure of the BH3 domain (shown as *) paralleled by a decreased exposure of the pocket region in human ALS.