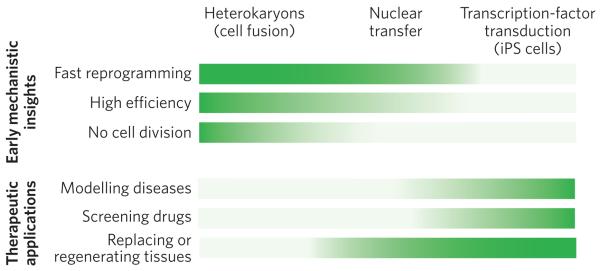
Figure 5. Comparison of the advantages of the three approaches to nuclear reprogramming.
The three approaches to reprogramming somatic cells differ in their technical difficulty, speed of reprogramming, efficiency of inducing pluripotency, and cell yield. Therefore, each approach is better suited for studies that provide early mechanistic insights (top) or for therapeutic applications (bottom). The greater the intensity of the colour, the more advantageous the technique. For gaining mechanistic insights (top) into the onset of reprogramming, heterokaryons are particularly advantageous, for three main reasons. First, they are quickly reprogrammed to express pluripotency genes (1 to 2 days ). This is also the case for nuclear transfer. By contrast, it takes weeks to generate iPS cells. Second, reprogramming by cell fusion is highly efficient. When mouse ES cells are fused with human fibroblasts, up to 70% of heterokaryons (enriched by fluorescence-activated cell sorting) activate the expression of pluripotency genes within 1 day. It is technically challenging (and therefore inefficient) to carry out nuclear transfer in mice, so it is difficult to use this approach for large-scale molecular analyses. Furthermore, the efficiency of generating iPS cells by transcription-factor transduction is low, about 0.01–0.1%. Third, cell division does not occur in heterokaryons. It also does not occur after nuclear transfer during the time when pluripotency genes are induced, allowing active mechanisms that induce pluripotency gene expression to be studied because this induction is independent of cell division and DNA replication; passive mechanisms may accompany cell division (for example dilution of DNA methyltransferases). By contrast, many rounds of cell division are required to generate iPS cells. For therapeutic applications (bottom), iPS cells are particularly advantageous, for three main reasons. First, diseases can readily be modelled using iPS cells derived from patients, overcoming the ethical issues and problems with immunological rejection that are inherent in obtaining human ES cells for studying disease. Skin fibroblasts can be readily obtained from the skin of an individual with a particular heritable disease, induced to become pluripotent in vitro and then induced to undergo differentiation to become the cell type of interest (for example a specific kind of cardiac cell). The pathways underlying a disease state (that is, gene expression and signalling) can thus be studied in cells that are not easily accessible in living humans. Second, drug screening can be carried out in vitro using these iPS-cell-based disease models to determine whether therapeutic drug candidates ameliorate or correct aberrant pathways. Third, for certain diseases, cell therapy might soon be used to regenerate or replace defective tissues, with the caveat that the tumorigenic potential, which is in part due to viral vector integration, must be overcome. Both nuclear transfer (leading to ES-cell production) and transcription-factor transduction (to produce iPS cells) have a high cell yield, which is important for cell therapy applications.