Abstract
We recently reported that prostate tumor-specific endothelial cells had features of mesenchymal stem cells and could trans-differentiate to form cartilage and bone-like tissues. Plasticity in the tumor vasculature may be related to well-known tumor blood vessel abnormalities and could underlie an intrinsic adaptive mechanism in tumor endothelial cells for circumventing anti-angiogenic strategies.
Keywords: Angiogenesis, tumor, endothelial cell, cancer, progenitor cell, stem cell
Introduction
It has been long- assumed that the blood vessels delivering blood, nutrients, and oxygen to tumors are just like normal ones. This premise has shaped the design of anti-angiogenic (anti-endothelial) drugs that can target and eliminate the tumor vasculature. There are now 10 anti-angiogenic drugs in clinical trials with many others in pre-clinical models 1. One of the more successful anti-angiogenic drugs already in the clinic is Avastin (Bevacizumab), an inhibitor of vascular endothelial growth factor (VEGF), which is approved for treating colorectal and lung cancers. However, the long-term efficacy of anti-angiogenic therapies for shrinking tumors is yet to be determined. The success of anti-angiogenic therapies rests on our ability to design rational anti-angiogenic strategies that are not based on the assumption that normal endothelial cells (EC) are identical to tumor-specific endothelial cells (TEC). To do this, we should continue to take advantage of new technologies that allow us to specifically isolate TEC and rigorously characterize them to learn more about the biology of these cells compared to their normal counterparts.
Normal blood vessels are usually comprised of a single layer of EC surrounded by perivascular cells called pericytes. EC turnover in vivo is normally slow. But EC can be rapidly activated when needed and the turnover may be 20 – 2000 times the rate in quiescent tissues 2. For example during wound healing, new blood vessels arise due to sprouting of the pre-existing “adult” EC or due to expansion of highly proliferative endothelial progenitor cells (EPC). EPC may be recruited from the circulation or may reside in the vessel wall 3. A hallmark of angiogenesis is the increased permeability of blood vessels. VEGF (also known as vascular permeability factor, VPF) released by mural cells and inflammatory cells both stimulates the proliferation and migration of EC and contributes to hyper-permeability in the endothelium. Therefore, the leakage of plasma proteins and fibrin is common in angiogenic blood vessels. But this process is transitory and reversible. Once the tissue remodeling that accompanies wound healing is completed, multiple mechanisms are in place to restore homeostasis.
In tumors, the homeostatic mechanisms that regulate angiogenesis are impaired. As a result, tumor blood vessels are abnormal and dysfunctional. Tumor blood vessels are tortuous, leaky, and lack a normal hierarchical arrangement 4. The basement membrane may be abnormally layered, discontinuous, or absent and the pericytes are loosely attached. These morphological abnormalities in tumor blood vessels have been captured by striking images 5. Some of the abnormalities in tumor blood vessels may be reversible (normalization) following treatment with anti-angiogenic drugs 6. By isolating TEC from different tumors, our knowledge about the biology of these cells has grown substantially. For a long time this was difficult because tumors are complex “organs” made up of many different cell types. However, St. Croix et al. published one of the first comprehensive molecular studies of TEC using serial analysis of gene expression (SAGE) on magnetic bead-selected colon TEC 7. Several TEC-specific markers were identified and were named tumor endothelial markers (TEMs). Multiple studies have followed and have confirmed both cytogenetic 8 and epigenetic 9 alterations in isolated TEC and other tumor stromal cells compared to their normal counterparts. Recently, Ghosh et al. showed that cultured TEC from prostate tumors failed to reorient their actin cytoskeleton to uniaxial cyclic strain - one of the first reported functional differences in TEC compared to normal EC 10.
We recently reported that prostate TEC from TRAMP mice (Transgenic Adenocarcinoma of the Mouse Prostate) expressed the expected EC markers such as CD31 and VE-Cadherin but also had features of mesenchymal stem cells (MSC) 11. Prostate TEC had a mesenchymal-like appearance in culture and alkaline phosphatase activity was inducible after stimulation with osteogenic medium. After prolonged culture in osteogenic medium (3–4 weeks), TEC underwent mineralization (calcification) and expressed bone-specific markers such as osteopontin and osteocalcin. TEC also differentiated to form cartilage-like tissues when cultured in chondrogenic medium and up-regulated the expression of cartilage-specific col 2a1 and sox 9. TEC did not differentiate to form adipocytes, which distinguishes these cells from bona fide MSC. In vivo, ectopic vascular calcification was detected at the luminal surface in about 4 % of human prostate tumor specimens. Thus, at least in prostate tumors, TEC can be characterized as having properties of MSC with the ability to differentiate to form cartilage and bone-like tissues.
Potential mechanisms underlying the apparent mesenchymal-like differentiation of TEC were recently highlighted 12. One possibility is that bona fide vascular EC are dedifferentiated into cells with mesodermal properties similar to the dedifferentiation of epithelial cells in tumors. The result would be an endothelial-to-mesenchymal transition and reversion to a more primitive state. On the other hand, mesodermal progenitors recruited from the circulation or local environment to the vascular bed in tumors may be differentiated to form EC while retaining some mesodermal properties. Mesodermal cell types such as MSC normally differentiate to form bone, fat, cartilage and other connective tissues. However, direct trans-differentiation of MSC into EC has been described 13 and a common ancestor for EC and pericytes has been postulated 14. Multi-potent mesodermal progenitors with vascular potential have also been described in hemangioma 15 and renal carcinoma 16. Similar to EPC, MSC residing in the perivascular space may act as a reserve for pericytes (and possibly EC) when needed but differentiation into vascular cells would require the right combination of growth factors. In this scenario, tumor-mediated factors would control progenitor cell differentiation but the expression of these factors may be tumor-type dependent. Thus, the differentiation fate and plasticity of vascular progenitors may differ from tumor-to-tumor and would depend on which factors are present in the tumor microenvironment (figure 1A).
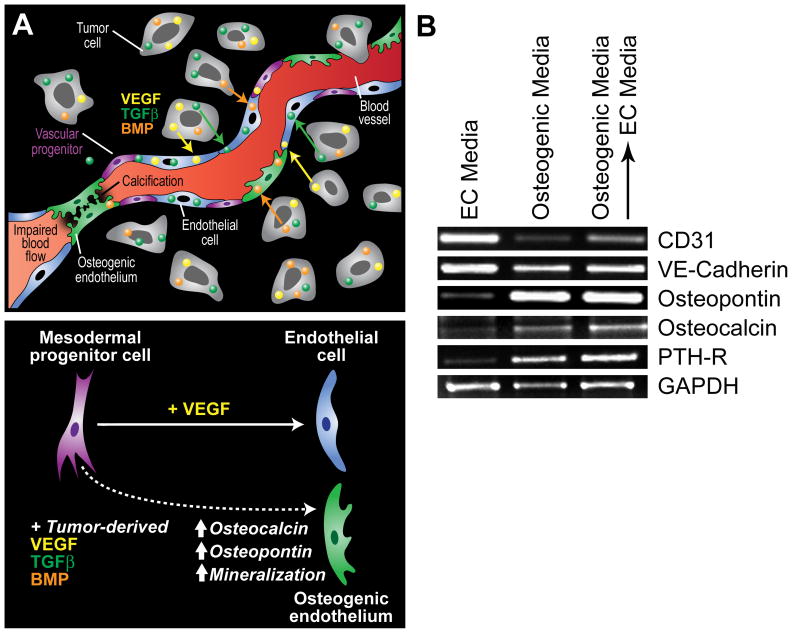
Figure 1.
Potential mechanisms contributing to ectopic calcification in tumor blood vessels. (A) In prostate cancer, tumor cells often express factors that support osteoblast proliferation such as VEGF, BMP, TGFβ, and IGF. Because mesenchymal and vascular progenitor cells are present in the tumor microenvironment, especially in the perivascular niche within the tumor stroma, tumor-specific expression of osteogenic factors may actively drive their pathological differentiation into bone or cartilage (top). Calcification in tumor blood vessels may impair cell-to-cell contact between adjacent endothelial cells due to loss of cellular adhesion molecules allowing for tumor cell intravasation across the blood vessel wall (upper right of figure). Luminal calcification may also impair blood flow creating hypoxic regions within the tumor microenvironment (lower left). While mesodermal progenitors may be induced to differentiate to form bone fide endothelium in the presence of VEGF, the cocktail of bone and vascular differentiation factors in the tumor microenvironment may result in an osteogenic endothelium (bottom). (B) The inducible expression of bone-specific markers in TEC may not be reversible. In the experiment, TEC from prostate tumors were cultured in EC media, osteogenic media (three weeks), or osteogenic media for three weeks followed by a return to EC media for an additional three weeks. Vascular and bone-specific markers were measured by RT-PCR. While the EC markers CD31 and VE-cadherin were down-regulated by prolonged culture in osteogenic media, their levels were increased after returning cells to EC media. On the other hand, osteopontin, osteocalcin, and parathyroid hormone receptor (PTH-R) were up-regulated by osteogenic media but was not reversible after returning cells to EC media.
What is the significance of TEC plasticity? Plasticity in the vasculature (endothelial-to-mesenchymal transition) is well-documented in pulmonary arteries 17 and tumor blood vessels 18. EC are constantly exposed to fluctuations in hormones, peptides, and other soluble factors carried by the blood, so these cells must adapt quickly when needed, for example during mechanical injury when blood flow is altered and pro-inflammatory cytokines are released. After return to homeostasis, reversal of the mesenchymal program may result in a reacquisition of endothelial characteristics. But in the tumor microenvironment, a unique cocktail of factors released by tumor cells may drive the differentiation of multi-potent progenitor cells residing in the vessel wall resulting in atypical or pathological differentiation into bone or cartilage like tissues. MSC are present in tumors 19 and are indistinguishable from pericytes 20. Their tumor-mediated differentiation could account for the unusual presence of calcifications reported in the stroma of breast and other tumors 21. Whether or not this is a reversible process or is significant to tumor progression is not clear, but in vitro studies in our laboratory suggest that the inducible expression of osteogenic factors by TEC is not reversible because both endothelial and osteocytic markers continue to be co-expressed even after return to endothelial-specific medium (figure 1B).
There are many unanswered questions requiring further investigation. One, it is not clear whether tumor blood vessel calcification is a property of other tumors. Breast tumors, for example, are often characterized by calcified nodules and medial calcification in arteries, but microvascular-specific calcifications in these tumors has not been reported 22. Two, does vascular calcification relate to the already-known abnormalities in tumor blood vessels such as altered blood flow and vessel contraction? Ectopic bone, fat, and cartilage have been described in diseased blood vessel walls for many years and probably contribute to vascular stiffness and hypertension. Three, endothelial-to-mesenchymal transition is usually accompanied by loss of cell-to-cell adhesion molecules such as VE-cadherin. Could this process in tumor blood vessels facilitate the intravasation of tumor cells into the blood stream by impairing the structural integrity of blood vessels? Finally, why is calcification luminal in tumor blood vessels, as opposed to the typical medial calcification in atherosclerotic arteries where calcifying vascular cells similar to MSC/pericytes undergo mineralization 23? Multiple, closely-related cell types from the vessel wall may undergo osteoblastic differentiation. The location of ectopic vascular calcification may depend on the cell’s position within the vascular wall (perivascular versus luminal). It may also be that the mechanisms and soluble factors that regulate blood vessel calcification in atherosclerosis are entirely different when compared to tumors.
Perspective
As studies continue to highlight the differences in TEC compared to their normal counterparts, the success of anti-angiogenic strategies for treating cancer first championed by Dr. Judah Folkman will depend on thoroughly understanding the biology of TECs. As recently reviewed by Bergers and Hanahan, the benefits of anti-angiogenic therapies in the clinic have been transitory and characterized by restoration of tumor growth following a period of regression 24. Are there mechanisms in place allowing TEC to adapt to and circumvent anti-angiogenic strategies? We predict that, just like tumor cells, TEC will differ depending on the tumor microenvironment and perhaps will vary during different stages of tumor progression (e.g. intraepithelial neoplasia versus adenocarcinoma). To overcome this heterogeneity in TEC, tumor-stage and tumor-type specific anti-angiogenic strategies may be needed that are thoughtfully designed based on empirical evidence, not on the assumption that TEC will behave identically to primary cultures of normal EC. To achieve this goal, high fidelity purification methods coupled with high throughput screening may help us identify a specific “molecular signature” in TEC from different tumors. For example, laser capture micro-dissection has now been used to “capture” blood vessels from breast 25 and prostate 26 tumors. These studies showed changes in the expression of over 1000 genes in breast tumor blood vessels and promoter methylation of GSTP1 and RARbeta2 in prostate tumor blood vessels when compared to their normal counterparts. The challenge will be to use this information to develop rational anti-angiogenic strategies that can hopefully translate into a demonstrable benefit in the clinic.
Acknowledgments
This work was supported by NIH grants CA37392 and CA45548. ACD wishes to thank Leonora DeBella and the American Cancer Society for supporting his research with a post doctoral fellowship. We thank Kristin Johnson for her excellent assistance with the figures.
References
- 1.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–86. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 2.Hobson B, Denekamp J. Endothelial proliferation in tumours and normal tissues: continuous labelling studies. Br J Cancer. 1984;49:405–13. doi: 10.1038/bjc.1984.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783–6. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 4.Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15:102–11. doi: 10.1016/j.gde.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 5.McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med. 2003;9:713–25. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- 6.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 7.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197–202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 8.Hida K, Hida Y, Amin DN, Flint AF, Panigrahy D, Morton CC, Klagsbrun M. Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res. 2004;64:8249–55. doi: 10.1158/0008-5472.CAN-04-1567. [DOI] [PubMed] [Google Scholar]
- 9.Hu M, Yao J, Cai L, Bachman KE, van den Brule F, Velculescu V, Polyak K. Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet. 2005;37:899–905. doi: 10.1038/ng1596. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh K, Thodeti CK, Dudley AC, Mammoto A, Klagsbrun M, Ingber DE. Tumor-derived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proc Natl Acad Sci U S A. 2008;105:11305–10. doi: 10.1073/pnas.0800835105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudley AC, Khan ZA, Shih SC, Kang SY, Zwaans BM, Bischoff J, Klagsbrun M. Calcification of multipotent prostate tumor endothelium. Cancer Cell. 2008;14:201–11. doi: 10.1016/j.ccr.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verfaillie CM. Bony endothelium: tumor-mediated transdifferentiation? Cancer Cell. 2008;14:193–4. doi: 10.1016/j.ccr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Riha GM, Yan S, Li M, Chai H, Yang H, Yao Q, Chen C. Shear stress induces endothelial differentiation from a murine embryonic mesenchymal progenitor cell line. Arterioscler Thromb Vasc Biol. 2005;25:1817–23. doi: 10.1161/01.ATV.0000175840.90510.a8. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K, Nishikawa S. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–6. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 15.Khan ZA, Boscolo E, Picard A, Psutka S, Melero-Martin JM, Bartch TC, Mulliken JB, Bischoff J. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J Clin Invest. 2008;118:2592–9. doi: 10.1172/JCI33493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruno S, Bussolati B, Grange C, Collino F, Graziano ME, Ferrando U, Camussi G. CD133+ renal progenitor cells contribute to tumor angiogenesis. Am J Pathol. 2006;169:2223–35. doi: 10.2353/ajpath.2006.060498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1–8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- 18.Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer. 2008 doi: 10.1038/sj.bjc.6604662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 20.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Guinebretiere JM, Menet E, Tardivon A, Cherel P, Vanel D. Normal and pathological breast, the histological basis. Eur J Radiol. 2005;54:6–14. doi: 10.1016/j.ejrad.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Tse GM, Tan PH, Pang AL, Tang AP, Cheung HS. Calcification in breast lesions: pathologists’ perspective. J Clin Pathol. 2008;61:145–51. doi: 10.1136/jcp.2006.046201. [DOI] [PubMed] [Google Scholar]
- 23.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–48. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhati R, Patterson C, Livasy CA, Fan C, Ketelsen D, Hu Z, Reynolds E, Tanner C, Moore DT, Gabrielli F, Perou CM, Klauber-DeMore N. Molecular characterization of human breast tumor vascular cells. Am J Pathol. 2008;172:1381–90. doi: 10.2353/ajpath.2008.070988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grover AC, Tangrea MA, Woodson KG, Wallis BS, Hanson JC, Chuaqui RF, Gillespie JW, Erickson HS, Bonner RF, Pohida TJ, Emmert-Buck MR, Libutti SK. Tumor-associated endothelial cells display GSTP1 and RARbeta2 promoter methylation in human prostate cancer. J Transl Med. 2006;4:13. doi: 10.1186/1479-5876-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]