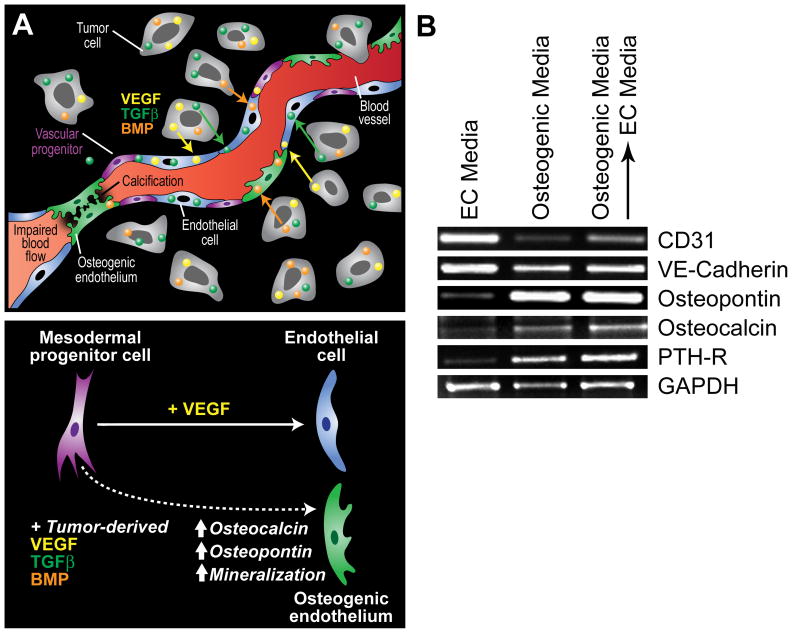
Figure 1.
Potential mechanisms contributing to ectopic calcification in tumor blood vessels. (A) In prostate cancer, tumor cells often express factors that support osteoblast proliferation such as VEGF, BMP, TGFβ, and IGF. Because mesenchymal and vascular progenitor cells are present in the tumor microenvironment, especially in the perivascular niche within the tumor stroma, tumor-specific expression of osteogenic factors may actively drive their pathological differentiation into bone or cartilage (top). Calcification in tumor blood vessels may impair cell-to-cell contact between adjacent endothelial cells due to loss of cellular adhesion molecules allowing for tumor cell intravasation across the blood vessel wall (upper right of figure). Luminal calcification may also impair blood flow creating hypoxic regions within the tumor microenvironment (lower left). While mesodermal progenitors may be induced to differentiate to form bone fide endothelium in the presence of VEGF, the cocktail of bone and vascular differentiation factors in the tumor microenvironment may result in an osteogenic endothelium (bottom). (B) The inducible expression of bone-specific markers in TEC may not be reversible. In the experiment, TEC from prostate tumors were cultured in EC media, osteogenic media (three weeks), or osteogenic media for three weeks followed by a return to EC media for an additional three weeks. Vascular and bone-specific markers were measured by RT-PCR. While the EC markers CD31 and VE-cadherin were down-regulated by prolonged culture in osteogenic media, their levels were increased after returning cells to EC media. On the other hand, osteopontin, osteocalcin, and parathyroid hormone receptor (PTH-R) were up-regulated by osteogenic media but was not reversible after returning cells to EC media.