Abstract
Calls for the eradication of malaria require the development of global and regional strategies based on a strong and consistent evidence base. Evidence from the previous global malaria eradication program and more recent transborder control campaigns have shown the importance of accounting for human movement in introducing infections to areas targeted for elimination. Here, census-based migration data were analyzed with network analysis tools, Plasmodium falciparum malaria transmission maps, and global population databases to map globally communities of countries linked by relatively high levels of infection movements. The likely principal sources and destinations of imported cases in each region were also mapped. Results indicate that certain groups of countries, such as those in West Africa and central Asia are much more strongly connected by relatively high levels of population and infection movement than others. In contrast, countries such as Ethiopia and Myanmar display significantly greater isolation in terms of likely infection movements in and out. The mapping here of both communities of countries linked by likely higher levels of infection movement, and “natural” migration boundaries that display reduced movement of people and infections between regions has practical utility. These maps can inform the design of malaria elimination strategies by identifying regional communities of countries afforded protection from recolonization by surrounding regions of reduced migration. For more isolated countries, a nationally focused control or elimination program is likely to stand a better chance of success than those receiving high levels of visitors and migrants from high-transmission regions.
Keywords: eradication, migration, network analysis, imported malaria, community detection
Significant progress is being made in reducing the morbidity and mortality attributed to malaria globally (1–10), encouraging the Global Malaria Action Plan (GMAP) (11) to articulate a long-term vision for malaria eradication through shorter-term local efforts to eliminate malaria. A total of 34 of the 107 malaria endemic countries have declared they have a national policy for malaria elimination or are pursuing spatially progressive elimination within their borders (11–13).
The most resource-efficient strategies for regional malaria elimination and global eradication will likely involve prioritization of resources in some regions or countries and the execution of a strategically planned and spatially progressive wave of elimination. Countries in low-transmission settings will face difficulties in (i) achieving elimination and, if they are successful, (ii) maintaining malaria-free status if they face a steady stream of incoming malaria cases from neighbors that continue to have high transmission. In fact, of the 25 countries in which malaria was eliminated during the Global Malaria Eradication Program (GMEP) of the 1950s, with the exception of Chile and Israel, all were either islands or contiguous with other countries that also eliminated malaria (14).
Human populations frequently move across national borders, and national malaria control effectiveness can be compromised by imported malaria (15). Imported malaria cases carry parasites, including resistant strains, even when asymptomatic (16). Illustrative examples of such issues include Zimbabwe, which had interrupted transmission in the 1960s but now has stable endemic transmission once again (17–19), and Vietnam, which achieved great successes against malaria during the GMEP, but saw these gains lost during the 1980s (1). In both instances, the combination of weaker malaria control and rapid invasion of malaria from imported cases rolled back significant gains against the disease. Despite the importance of cross-border malaria dynamics, efforts to control or eliminate malaria have generally been approached on a country-by-country basis. Loans or grants are generally negotiated with single countries based on plans that are disconnected from those of neighboring countries, treating malaria control in a country as insulated from malaria control activities in neighboring countries (20). Recent, more successful regional efforts, such as the Lubombo Spatial Development Initiative (LSDI) (21) have, however, demonstrated the importance of transborder approaches to malaria control. Understanding the rates of migration and parasite movement within and between regions therefore has implications for optimizing the strategic deployment of malaria control internationally.
Mapping “natural” migration boundaries that display reduced movement of people and infections between regions has practical utility. These maps enable malaria interventions to be applied and coordinated in regional blocks afforded protection from recolonization by surrounding regions of reduced migration. In this study, a recently constructed bilateral international migration database was analyzed with network analysis tools in combination with Plasmodium falciparum malaria transmission maps and global population databases to infer globally communities of countries linked by relatively strong population movement and imported case flows. Net contributors and receivers of imported cases were then quantified and highlighted, and the prospects for control and elimination by region discussed.
Results
P. falciparum Migration Network Communities.
Figs. 1–3 show results of regional community structure analyses based on migration data, P. falciparum malaria transmission maps, and global population databases (Figs. S1–S3 show results based solely on migration and population databases). The maps highlight those countries that form communities linked by high levels of population movement from high transmission areas. In each case, a graph showing the relative strength of the communities found (measured by modularity; Materials and Methods) for each step of merging countries into new communities is presented, along with maps showing the communities identified at significant points in this merging process. SI Text describes a similar analysis based solely on migration data.
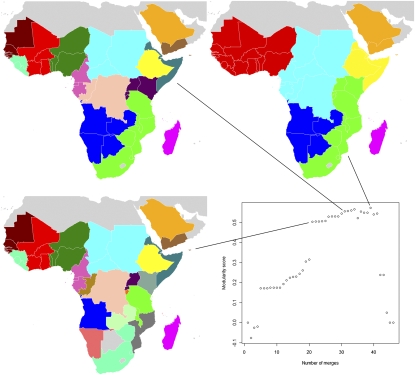
Fig. 1.
P. falciparum migration communities for the Africa and Arabian Peninsula region. Each map represents a different stage of country-merging into communities connected by relatively higher levels of infection movements than to the surrounding regions, with community membership shown by color. For instance, the bottom-left map shows that Mali, Burkina Faso, Côte d'Ivoire, and Ghana form a community (colored red). The plot in the center shows the overall strength (measured by modularity score) of clustering at different stages of merging countries together into communities. The stage that each map shown represents is identified by the connecting lines.
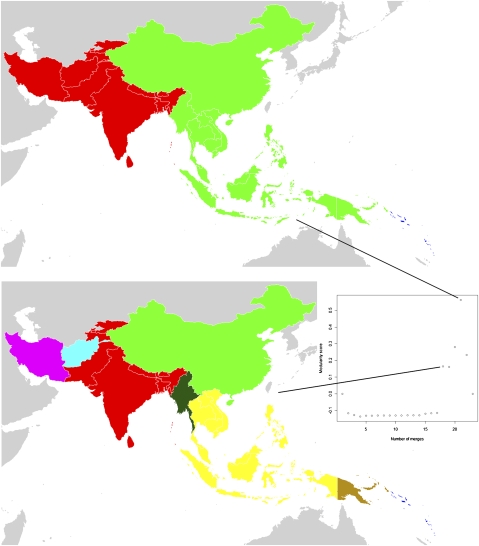
Fig. 3.
P. falciparum migration communities for the Central and Southeast Asia region. Each map represents a different stage of country-merging into communities connected by relatively higher levels of infection movements than to the surrounding regions, with community membership shown by color. For instance, the top map shows that the Solomon Islands and Vanuatu form a community (colored dark blue). The plot in the center-right shows the overall strength (measured by modularity score) of clustering at different stages of merging countries together into communities. The stage that each map shown represents is identified by the connecting lines.
Fig. 1 shows that intercountry infection movement is likely more prevalent in some areas of the Africa and Arabian Peninsula region than others. Throughout the agglomeration steps shown, communities of countries in West Africa (in red and dark green), and North-Central Africa (in cyan) are consistently found, highlighting the greater levels of infection exchange, and circulation between these countries than to the rest of the region. In contrast, other countries remain isolated, even at some of the highest levels of agglomeration. Madagascar, Ethiopia, and Saudi Arabia exhibit no distinct membership to a network community within the Africa and Arabian Peninsula area until the final agglomeration steps, indicating overall low levels of infection movement in and out to the remainder of the region.
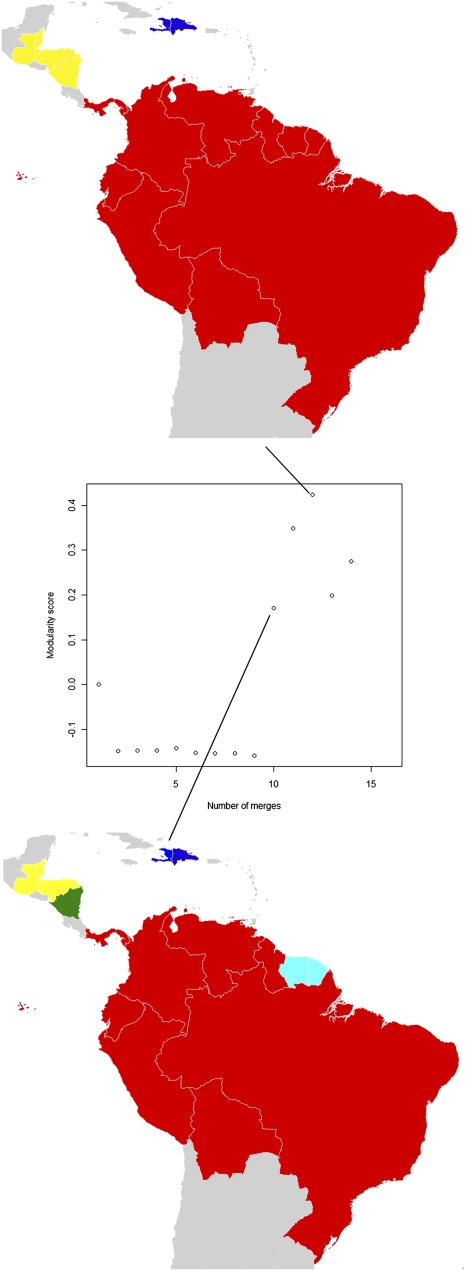
The Americas display a range of community structures (Fig. 2), with the highest modularity score defining three communities: (i) the Central American P. falciparum malaria endemic countries, (ii) continental South America, and (iii) Hispaniola. The low modularity scores for earlier iterations shows that smaller communities of countries linked by strong migrant exchange are not such a feature of the Americas as was seen for the Africa and Arabian Peninsula region.
Fig. 2.
P. falciparum migration communities for the Americas region. Each map represents a different stage of country-merging into communities connected by relatively higher levels of infection movements than to the surrounding regions, with community membership shown by color. For instance, the top map shows that the Suriname and French Guiana form a community (colored cyan). The plot in the center shows the overall strength (measured by modularity score) of clustering at different stages of merging countries together into communities. The stage that each map shown represents is identified by the connecting lines.
Fig. 3 shows that the community configuration for Central and Southeast (CSE) Asia that results in the largest modularity score is a division into three distinct and spatially contiguous regions representing West Asia, East Asia, and the isolated islands of Vanuatu and the Solomons. It is the earlier stages of the agglomeration process that reveals interesting within-region structures in terms of countries connected by relatively high levels of infection movement. For instance, the set of communities shown in the bottom map highlights the relative isolation of Papua New Guinea, Myanmar, China, Iran, and Afghanistan, with each displaying no community membership. In contrast, in central East Asia it can be seen that Vietnam, Cambodia, Laos, Thailand, Indonesia, Malaysia, and the Philippines are strongly connected to each other.
Migration and P. falciparum Malaria Sources and Sinks.
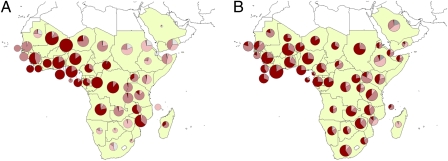
Fig. 4 shows which nations are likely net exporters (sources) of P. falciparum parasites and which are net importers (sinks) in the Africa and Arabian Peninsula region, and in combination with Fig. 1, this map describes general patterns of infection movements. Movements are categorized by their origin PfPR2–10 (Plasmodium falciparum Parasite Rate in the 2–10 year age group) endemicity class, as defined by Hay et al. (22). Here we focus on examining the control relevant situations of movements from high-transmission (PfPR2–10 > 40%) to low-transmission regions (PfPR2–10 < 40%). SI Text describes the results for the Americas and CSE Asia regions.
Fig. 4.
Africa and Arabian Peninsula. (A) Outgoing migrants and (B) incoming migrants by endemicity class. Endemicity classes are colored as: gray, unstable risk; light pink, PfPR2–10 = 0–5%; midpink, PfPR2–10 = 5–40%; and dark red, PfPR2–10 = >40%. Pie chart size is representative of the relative number of migrants.
Within the Africa and Arabian Peninsula region, it can be seen that relatively high levels of migration occur across continental Africa, with some of the largest numbers of migrants coming from the most intensely malarious regions (PfPR2–10 > 40%). This results in a general pattern of infection movements out of the high-transmission cores of West and Central Africa (Fig. 4A) into the surrounding lower-transmission countries (Fig. 4B). The largest migrant sources are found in Sahelian West Africa, with Mali, Burkina Faso, and Niger being net migrant exporters, principally to their neighboring coastal West African countries within the strongly linked migration community identified in Fig. 1. With relatively high migration levels also back the other way, this likely results in an ongoing exchange of P. falciparum infections, with the lower-transmission countries of Senegal, Mauritania, and Niger, for instance, receiving a higher proportion of migrants from the highest-transmission regions than they provide as migrant sources.
Focusing on the PfPR2–10 > 40% class again, Angola and Mozambique represent the principal sources of migrants from high-transmission areas to low-transmission areas within the southern Africa communities (Fig. 1). However, with the database used here representing foreign-born and foreign-national populations, these figures are potentially biased, reflecting outmigration due to the conflicts in Angola and Mozambique. With these conflicts subsiding, movements may also be decreasing.
The north-central migration community identified in Fig. 1 covers the full range of P. falciparum endemicity classes. Fig. 4 A and B show how large population flows from the high-transmission countries of the Democratic Republic of Congo, Congo, and Central African Republic northward and eastward are likely resulting in the lower transmission countries in the region receiving a high proportion of their incoming migrants from these regions.
Discussion
The first malaria eradication campaigns showed that movement of malaria parasites by human migration can quickly undermine the successful interruption of transmission (23). Malaria control has once again risen up the international agenda over the past decade, so much so that numerous countries have made elimination a national aim, and global eradication is being discussed once again (11, 24). Since the GMEP, the concepts of receptivity and vulnerability have been used in assessing whether malaria elimination is feasible in an area (25), but attempts to quantify both have only recently been made (26). High vulnerability, exhibited principally by the movement of people and, consequently, infections means that in many cases a multicountry transborder approach to control and elimination is favorable over one focused at the national level. Here, the identification of communities of countries linked by high levels of movement and their composition of sources and sinks of imported P. falciparum infections provides a first basic step toward evidence-based and globally comparable measures of vulnerability.
Findings indicate that certain communities of countries, such as those in West Africa and Central Asia are more strongly connected by high levels of infection movement than others. The effects of this can be seen for instance in Bhutan, where 77% of all its malaria cases originate in three districts located on its southern border with India (12). Efficient control and elimination strategies for such communities of countries should consider transborder coordination, rather than solely nationally focused aims. Examples of where this is being initiated include the Asia Pacific Malaria Elimination Network (27) and the E8 group of eight southern African countries (28). Interestingly, Fig. 1 suggests that this initiative could be more effectively reformulated into east and west divisions.
In Africa, the pattern of communities identified mirrors, to an extent, the distribution of antimalarial drug resistance lineages (29). This provides further evidence of the probable central role of migration in the regional dispersal of resistant malaria, and the need for further studies on human movement patterns around the world to design strategies for the control of any artemisinin-resistant strains that may arise.
Some countries display significantly greater isolation in terms of likely P. falciparum infection movements in and out relative to the majority of countries. Unsurprisingly, these include, for instance, the island nations of Vanuatu, Solomon Islands, and Madagascar, where transport to/from them involves much greater costs in terms of time and expense. However, other countries display a more surprising level of isolation, including Ethiopia, where both geographical and cultural factors may inhibit strong cross-border movement, and Myanmar, where reduced movement is principally related to political restrictions and poor infrastructure. If it were to be considered, a nationally focused control or elimination program for these nations is likely to stand a better chance of success than those receiving high levels of visitors and migrants from high-transmission regions.
In terms of the control and elimination of P. falciparum malaria, Fig. 4A (see also Figs. S4A and S5A) highlights how certain countries, such as Burkina Faso and Central African Republic, represent likely sources of significant numbers of cases exported to neighboring countries. Concerted control efforts in these “source” nations will not only reduce the burden of P. falciparum in the nations themselves, but should also have substantial knock-on effects in reducing case numbers and burden in lower-transmission “sink” countries that are within their migration communities. Nevertheless, such factors do highlight the need for countries considering embarking on an elimination campaign to conduct full assessments of human movement patterns and rates from surrounding countries within their migration-linked regional communities (Figs. 1–3) that form likely sources of imported malaria (see Fig. 4 and SI Text).
The migration data used here have some significant limitations for drawing conclusions at national to local scales (Materials and Methods). Thus, though these analyses present a starting point and evidence base for the analysis of global and regional patterns of the relative levels of parasite movements between countries, they certainly provide no replacement for detailed assessments of human movement patterns, and should not be interpreted beyond relative measures of regional movements. In the new era of elimination it will be important to understand the forces that govern parasite migration, and a wide range of datasets, techniques, and tools now exist for quantifying human movement patterns across a range of spatial and temporal scales (30–34), and ultimately provide validation for some of the assumptions made here. Moreover, modeling frameworks are being developed in an effort to aid understanding the effects of movement upon transmission (34, 35). The decision to embark on elimination is multifactorial and is often not evidence based. Strategic assessments of elimination feasibility within a country or region require sophisticated analyses of information at spatial resolutions not easily summarized for global comparisons. A rigorous and structured study, covering quantitative and qualitative assessments of not only human and parasite movement patterns but also past and present epidemiology, health system adaptation, and financial sustainability should be undertaken, as was done recently for Zanzibar (26).
The impact of control interventions will always be maximized when applied at a geographical scale that encompasses regions of significant volumes of parasite exchange. Coordinated campaigns within regions connected by significant population movements from high-transmission areas will be more likely to succeed than campaigns within defined national territories, which will face an uphill struggle against the importation of malaria.
Materials and Methods
Quantifying Relative Levels of Human Movement.
A global analysis of likely malaria infection movements should ideally be based upon subnational data on population flows, capturing the full range of relevant movements (30, 34, 36). However, such data are nonexistent for the vast majority of countries (particularly malaria-endemic countries), and both patchy and extremely variable for the remainder of countries, even highly developed countries. Moreover, where it does exist, it is almost always incomparable between countries. Foreign-born and foreign-national population data derived from recent censuses represent the most complete and comparable datasets for global and regional analyses that most readily accord with actual population movements (37), and these were used here as a measure of the relative levels of movement between countries.
Data on international bilateral migrant stocks for 226 countries and territories in 2000–2002 were obtained (37). Wherever possible, these data were derived from the latest round of censuses, as these were considered most comparable at the global level. Where unavailable, population registers were drawn upon, and in the cases of missing data, a variety of techniques and tests were used to create and validate a complete matrix of international bilateral migrant stocks (37). Finally, all data before 2000 were scaled to the United Nations midyear totals of migrant stocks for 2000 (38). For each country or territory, the completed dataset represented the number of foreign-born and foreign-nationality people in residence in 2000–2002, and which country/territory they were born in or had come from. These data do not capture both very short-term and illegal movements, which themselves can be substantially larger than those in official records (see, e.g., ref. 39).
The migrant dataset was rescaled to account for the population size of each country of origin, providing a measure of the strength of migration between countries. For instance, a country containing 100,000 residents born in country A (population 1 million) and 100,000 residents born in country B (population 10 million) has a stronger migratory pull to country A than B. National population totals of each country were obtained (40), and used to convert the migrant stock numbers into percentages of national origin country population.
Global P. falciparum malaria endemicity data were obtained from the P. falciparum malaria maps (22) recently released by the Malaria Atlas Project (MAP; www.map.ox.ac.uk/). These data were used in combination with gridded population data to obtain measures of population-weighted transmission for each country, as measured by the P. falciparum parasite rate in the 2–10 age group (PfPR2–10) (41). The gridded global population data were obtained from the Global Rural Urban Mapping Project (GRUMP) gridded population surface (42), supplemented with more spatially detailed data from the AfriPop project (www.afripop.org/) (43), where available. Finally, the data outlining control-relevant classes of P. falciparum transmission (22, 41) were also obtained.
Analyses were stratified throughout by three major global regions: America; Africa, Yemen, and Saudi Arabia (Africa and the Arabian Peninsula); and Central and South and East Asia (CSE Asia). This division allowed these biogeographically, entomologically, and epidemiologically distinct regions (44, 45) to be considered separately, and is further supported by the spatial structure exhibited by PfPR2–10 data (22).
P. falciparum Migration Network Communities.
The identification of groups or communities of countries linked by relatively high levels of population movement from high-transmission regions, equating to a likely greater level of imported infections into a destination country, first required the creation of a simple quantitative index to represent this. The P. falciparum-migration metric, Pfm, between country i and country j is defined as:
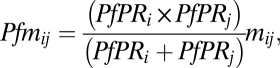 |
where mij is the rescaled migration from country i to country j. The index is highest if both (i) PfPR2–10 for country i and j are high, and (ii) the migration level is also high, thus representing large numbers of people moving between high-transmission countries, and large numbers of infection movements. The index is lowest if small numbers of migrants are moving between low-transmission countries, equating to little imported case exchange.
Network analysis approaches were applied to the matrix showing Pfm values between countries to identify well-defined communities of countries that are connected by relatively high levels of population movement from high-transmission regions. These communities were defined using random walk-based methodologies extended for weighted (46) and directed (47) networks and the strength of community linkage evaluated using measures of network modularity (48). In simple terms, at each stage of the analysis, the two countries (or communities of countries) that displayed the highest Pfm value between them were merged to form a new community. The strength of the new set of communities and individual countries in terms of Pfm values was then measured and recorded. These steps were repeated until all countries formed a single community. These steps are described in detail below.
The walktrap community structure algorithm was used to identify communities of countries that are most strongly linked by migration. The algorithm is based on the fact that random walks on a network tend to get trapped in strongly weighted parts corresponding to communities, and is described in detail in Pons and Latapy (46). A range of alternative algorithms exist for the detection of strongly connected communities within networks (e.g., refs. 48–50), and each of these were tested alongside the walktrap algorithm, but little change in results were found.
Network weights were calculated using methods outlined in Kim et al. (47) that account for directionality within a network. Initially, the set of n countries formed a weighted network of n communities, with the weights being the P. falciparum migration metrics, Pfm. Two communities (or countries, depending on the stage of the agglomeration), c1 and c2, were then chosen according to
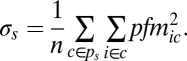 |
At each step, s, σs, a metric summarizing the strength of P. falciparum migration between communities, was calculated for every combination of neighboring community/country pairs possible, and the two communities (or countries) that maximized the mean σs of the squared Pfm between each country i and its community C were merged. This created a new community CZ and a new partition of the Pfm, Ps+1. Pfm values between communities were then updated and the procedure was repeated until n − 1 steps and partition Pn was obtained. Each step defined a partition Ps of the Pfm-weighted network into communities of countries, which provided a hierarchical structure of communities.
Quantifying and exploring which communities were the “strongest” in terms of P. falciparum exchange was undertaken by calculating, for each partition Ps from the walktrap algorithm, a network modularity score, Q(P). Modularity is a measure of the quality or strength of a given division of a network and is measured by
The best partition is considered to be the one that maximizes Q, where ec is the fraction of edges (links between countries) inside community C and ac is the fraction of edges bound to community C. Modularity is described in detail in Newman and Girvan (48). Here, the P. falciparum-migration community divisions for each world region that produced both the largest modularity scores and the largest increases in earlier steps between individual agglomerations were identified and mapped to visualize the strongest levels of P. falciparum exchange within communities. All approaches were implemented using the i-graph package (http://igraph.sourceforge.net/) within the R statistical computing environment (51).
Imported P. falciparum Case Origins and Destinations.
In the second part of the analyses, the relationship between the migrant stock database and P. falciparum malaria transmission data were used to explore global patterns in likely country-level origins and destinations of P. falciparum-carrying migrants. First, the global map of P. falciparum endemicity classes was overlaid onto the gridded population data to identify the proportions of people in each country or territory residing under each P. falciparum endemicity class. Assuming here that migrants are equally likely to come from any part of a country, the migrant database entries for outgoing migrants from each country were then split by the country's corresponding endemicity class proportions. This created five separate migrant matrices representing per-country migrant numbers outmigrating from no-risk, unstable risk, 0–5% PfPR2–10, 5–40% PfPR2–10, and >40% PfPR2–10, areas to all other countries. These matrices were mapped to show, for each country, the likely relative proportions and numbers of migrants from each PfPR class (i) outmigrating (imported case sources = contributing countries) and (ii) inmigrating (imported case sinks = receiving countries).
Supplementary Material
Acknowledgments
We thank Dr. Pete Gething and Dr. Simon Hay for comments on the original manuscript. This work forms part of the output of the AfriPop project (www.afripop.org/), supported by a grant from the Fondation Philippe Wiener–Maurice Anspach, and the Malaria Atlas Project (MAP; www.map.ox.ac.uk/), principally funded by the Wellcome Trust UK. A.J.T. and D.L.S. are supported by the Bill and Melinda Gates Foundation Grant 49446. D.L.S. also acknowledges funding support from the Research and Policy in Infectious Disease Dynamics program of the Science and Technology Directorate, Department of Homeland Security, and the Fogarty International Center, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002971107/-/DCSupplemental.
References
- 1.Barat LM. Four malaria success stories: How malaria burden was successfully reduced in Brazil, Eritrea, India, and Vietnam. Am J Trop Med Hyg. 2006;74:12–16. [PubMed] [Google Scholar]
- 2.Barnes KI, et al. Effect of artemether-lumefantrine policy and improved vector control on malaria burden in KwaZulu-Natal, South Africa. PLoS Med. 2005;2:e330. doi: 10.1371/journal.pmed.0020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattarai A, et al. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS Med. 2007;4:e309. doi: 10.1371/journal.pmed.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ceesay SJ, et al. Changes in malaria indices between 1999 and 2007 in The Gambia: A retrospective analysis. Lancet. 2008;372:1545–1554. doi: 10.1016/S0140-6736(08)61654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fegan GW, Noor AM, Akhwale WS, Cousens S, Snow RW. Effect of expanded insecticide-treated bednet coverage on child survival in rural Kenya: A longitudinal study. Lancet. 2007;370:1035–1039. doi: 10.1016/S0140-6736(07)61477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleinschmidt I, et al. Marked increase in child survival after four years of intensive malaria control. Am J Trop Med Hyg. 2009;80:882–888. [PMC free article] [PubMed] [Google Scholar]
- 7.Nyarango PM, et al. A steep decline of malaria morbidity and mortality trends in Eritrea between 2000 and 2004: The effect of combination of control methods. Malar J. 2006;5:33. doi: 10.1186/1475-2875-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okiro EA, et al. The decline in paediatric malaria admissions on the coast of Kenya. Malar J. 2007;6:151. doi: 10.1186/1475-2875-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Meara WP, et al. Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet. 2008;372:1555–1562. doi: 10.1016/S0140-6736(08)61655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teklehaimanot HD, Teklehaimanot A, Kiszewski A, Rampao HS, Sachs JD. Malaria in São Tomé and Principe: On the brink of elimination after three years of effective antimalarial measures. Am J Trop Med Hyg. 2009;80:133–140. [PubMed] [Google Scholar]
- 11.Roll Back Malaria. Global Malaria Action Plan. Geneva: Roll Back Malaria Partnership; 2008. [Google Scholar]
- 12.Feachem RGA, Phillips AA, Targett GA. Shrinking the Malaria Map: A Prospectus on Malaria Elimination. University of California, San Francisco: Global Health Group, Global Health Sciences; 2009. [Google Scholar]
- 13.World Health Organization. The World Malaria Report. Geneva: World Health Organization; 2008. [Google Scholar]
- 14.Brown AWA, Haworth J, Zahar AR. Malaria eradication and control from a global standpoint. J Med Entomol. 1976;13:1–25. doi: 10.1093/jmedent/13.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Prothero RM. Population movements and problems of malaria eradication in Africa. Bull World Health Organ. 1961;24:405–425. [PMC free article] [PubMed] [Google Scholar]
- 16.Wootton JC, et al. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature. 2002;418:320–323. doi: 10.1038/nature00813. [DOI] [PubMed] [Google Scholar]
- 17.Alves W. Malaria parasite rates in Southern Rhodesia: May–September 1956. Bull World Health Organ. 1958;19:69–74. [PMC free article] [PubMed] [Google Scholar]
- 18.Alves W, Blair DM. Malaria control in Southern Rhodesia. J Trop Med Hyg. 1955;58:273–280. [PubMed] [Google Scholar]
- 19.Mabaso ML, Sharp B, Lengeler C. Historical review of malarial control in southern African with emphasis on the use of indoor residual house-spraying. Trop Med Int Health. 2004;9:846–856. doi: 10.1111/j.1365-3156.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- 20.Snow RW, Guerra CA, Mutheu JJ, Hay SI. International funding for malaria control in relation to populations at risk of stable Plasmodium falciparum transmission. PLoS Med. 2008;5:e142. doi: 10.1371/journal.pmed.0050142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharp BL, et al. Seven years of regional malaria control collaboration—Mozambique, South Africa, and Swaziland. Am J Trop Med Hyg. 2007;76:42–47. [PMC free article] [PubMed] [Google Scholar]
- 22.Hay SI, et al. A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med. 2009;6:e1000048. doi: 10.1371/journal.pmed.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prothero RM. Migrants and Malaria. London: Longmans Green; 1965. [Google Scholar]
- 24.Feachem R, Sabot O. A new global malaria eradication strategy. Lancet. 2008;371:1633–1635. doi: 10.1016/S0140-6736(08)60424-9. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. Global Malaria Control and Elimination: Report of a Technical Review. Geneva: WHO; 2008. [Google Scholar]
- 26.Zanzibar Malaria Control Program. Malaria Elimination in Zanzibar: A Feasibility Assessment. Zanzibar, Tanzania: Zanzibar Ministry of Health and Social Welfare; 2010. [Google Scholar]
- 27.Hsiang MS, Abeyasinghe R, Whittaker M, Feachem RGA Malaria elimination in Asia-Pacific: an under-told story. Lancet. 2010;375:1586–1587. doi: 10.1016/S0140-6736(10)60350-9. [DOI] [PubMed] [Google Scholar]
- 28.Southern African Development Community (SADC) (2009) Elimination Eight (E8) Regional Initiative. Ministerial Resolution. Available at http://www.globalhealthsciences.ucsf.edu/pdf/E8MinResolution_20090303.pdf. Accessed June, 2010. [Google Scholar]
- 29.Pearce RJ, et al. Multiple origins and regional dispersal of resistant dhps in African Plasmodium falciparum malaria. PLoS Med. 2009;6:e1000055. doi: 10.1371/journal.pmed.1000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tatem AJ, et al. The use of mobile phone data for the estimation of the travel patterns and imported Plasmodium falciparum rates among Zanzibar residents. Malar J. 2009;8:287. doi: 10.1186/1475-2875-8-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vazquez-Prokopec GM, et al. Usefulness of commercially available GPS data-loggers for tracking human movement and exposure to dengue virus. Int J Health Geogr. 2009;8:68. doi: 10.1186/1476-072X-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.González MC, Hidalgo CA, Barabási AL. Understanding individual human mobility patterns. Nature. 2008;453:779–782. doi: 10.1038/nature06958. [DOI] [PubMed] [Google Scholar]
- 33.Osorio L, Todd J, Bradley DJ. Travel histories as risk factors in the analysis of urban malaria in Colombia. Am J Trop Med Hyg. 2004;71:380–386. [PubMed] [Google Scholar]
- 34.Stoddard ST, et al. The role of human movement in the transmission of vector-borne pathogens. PLoS Negl Trop Dis. 2009;3:e481. doi: 10.1371/journal.pntd.0000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cosner C, et al. The effects of human movement on the persistence of vector-borne diseases. J Theor Biol. 2009;258:550–560. doi: 10.1016/j.jtbi.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prothero RM. Disease and mobility: A neglected factor in epidemiology. Int J Epidemiol. 1977;6:259–267. doi: 10.1093/ije/6.3.259. [DOI] [PubMed] [Google Scholar]
- 37.Parsons CR, Skeldon R, Walmsley TL, Winters LA. Quantifying international migration: A database of bilateral migrant stocks. World Bank Policy Research Working Paper 4165. 2007 Available at http://econ.worldbank.org/ Accessed January, 2010. [Google Scholar]
- 38.United Nations. Trends in Total Migrant Stock 1960-2000. New York: United Nations Population Division; 2004. [Google Scholar]
- 39.Chamratrithirong A, Archavanitkul K, Richter K, Guest P, Thongthai V. National Migration Survey of Thailand. Bangkok: Mahidol University; 1995. [Google Scholar]
- 40.United Nations Population Division. World Population Prospects, 2008 Revision. New York: United Nations; 2008. [Google Scholar]
- 41.Hay SI, Smith DL, Snow RW. Measuring malaria endemicity from intense to interrupted transmission. Lancet Infect Dis. 2008;8:369–378. doi: 10.1016/S1473-3099(08)70069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balk DL, et al. Determining global population distribution: Methods, applications and data. Adv Parasitol. 2006;62:119–156. doi: 10.1016/S0065-308X(05)62004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tatem AJ, Noor AM, von Hagen C, Di Gregorio A, Hay SI. High resolution population maps for low income nations: Combining land cover and census in East Africa. PLoS ONE. 2007;2:e1298. doi: 10.1371/journal.pone.0001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macdonald G. The Epidemiology and Control of Malaria. London: Oxford Univ Press; 1957. [Google Scholar]
- 45.Mouchet J, et al. Biodiversité du Paludisme Dans le Monde. Montrouge, France: John Libbey Eurotext; 2004. [Google Scholar]
- 46.Pons P, Latapy M. Computing communities in large networks using random walks. Lect Notes Comput Sci. 2005;3733:284–293. [Google Scholar]
- 47.Kim Y, Son S-W, Jeong H. Link rank: Finding communities in directed networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2010;81:016103. doi: 10.1103/PhysRevE.81.016103. [DOI] [PubMed] [Google Scholar]
- 48.Newman MEJ, Girvan M. Finding and evaluating community structure in networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;69:026113. doi: 10.1103/PhysRevE.69.026113. [DOI] [PubMed] [Google Scholar]
- 49.Clauset A, Newman MEJ, Moore C. Finding community structure in very large networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;70:066111. doi: 10.1103/PhysRevE.70.066111. [DOI] [PubMed] [Google Scholar]
- 50.Reichardt J, Bornholdt S. Statistical mechanics of community detection. Phys Rev E Stat Nonlin Soft Matter Phys. 2006;74:016110. doi: 10.1103/PhysRevE.74.016110. [DOI] [PubMed] [Google Scholar]
- 51.R Development Core Team. Vienna: R Foundation for Statistical Computing; 2009. R: A Language and Environment for Statistical Computing. Available at http://www.R-project.org/ Accessed May, 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.