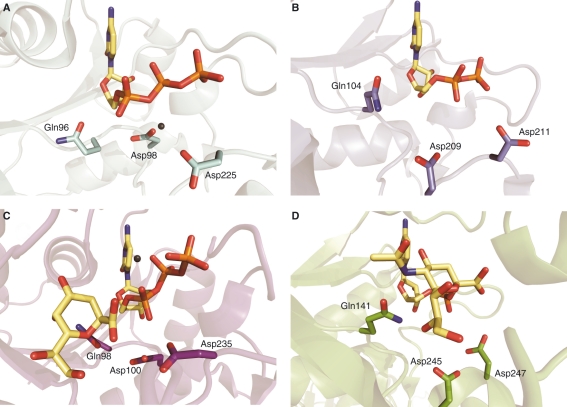
Fig. 3.
Metal-binding residues of CNS and related enzymes. (A) Gln96, Asp225 and Asp98 in the active site of K-CKS (cyan) (PDB 1GQ9) [13]; (B) Gln104, Asp211 and Asp209 in the Neisseria meningitidis enzyme (blue) (PDB 1EYR) [12]; (C) Gln98, Asp235 and Asp100 in L-CKS (purple) (PDB 3K8D) [14] (in addition, a second metal-binding site has been modelled in this enzyme coordinated by the β- and γ-phosphates of the CTP); and (d) the metal-binding residues deduced from the alignment in the structure of the murine CNS (green) (PDB 1QWJ) [18]. When present in the crystal structures, CTP, CDP, Kdo and CMP-Neu5Ac in the active sites are coloured by atom type with the carbon set as yellow. Mg2+ is coloured in grey.