Abstract
Summary: The epidemiology of Clostridium difficile infection (CDI) has changed dramatically during this millennium. Infection rates have increased markedly in most countries with detailed surveillance data. There have been clear changes in the clinical presentation, response to treatment, and outcome of CDI. These changes have been driven to a major degree by the emergence and epidemic spread of a novel strain, known as PCR ribotype 027 (sometimes referred to as BI/NAP1/027). We review the evidence for the changing epidemiology, clinical virulence and outcome of treatment of CDI, and the similarities and differences between data from various countries and continents. Community-acquired CDI has also emerged, although the evidence for this as a distinct new entity is less clear. There are new data on the etiology of and potential risk factors for CDI; controversial issues include specific antimicrobial agents, gastric acid suppressants, potential animal and food sources of C. difficile, and the effect of the use of alcohol-based hand hygiene agents.
INTRODUCTION
Since the description of Clostridium difficile as a cause of antimicrobial-associated diarrhea, colitis, and pseudomembranous colitis (PMC) in 1978, interest in this pathogen was predominantly because of its association with health care and its impact on morbidity and mortality in the elderly. There has been an explosion of reports on C. difficile infection (CDI) in the new millennium, driven by large increases in disease incidence, stark changes in clinical presentation and epidemiology, and descriptions of new risk factors. This review focuses on how these new issues have impacted the epidemiology of CDI.
A study of 187 isolates from multiple U.S. states revealed that a particular C. difficile strain was most closely associated with the increased incidence and altered clinical presentation of CDI. Prior to the year 2000, this strain (BI) had accounted for fewer than 1% of U.S. C. difficile isolates (130). The strain was characterized as toxinotype III and had an 18-bp deletion within the tcdC gene (putative negative regulator for the production of toxins A and B) as well as a deletion at position 117. The latter results in a frameshift and a premature stop codon, leading to a truncated TcdC protein. These strains have been shown to produce significantly more toxin in vitro, and it was postulated that this virulence factor is associated with increased severity (203), although other complex gut model data partly contradict these findings (68). In the gut model, this epidemic C. difficile strain (ribotype 027) produces toxin for longer periods of time, as opposed to more toxin per unit time, which may be expected considering the absence of a functional negative regulation mechanism (68). These strains have also been shown to produce binary toxin, although the role of this toxin in CDI pathogenesis remains largely unclear (75). A recent report mentioned that binary toxin induces microtubule protrusions that form a dense meshwork at the cell surface of intestinal epithelial cells, which wrap and embed bacterial cells, thereby increasing the adherence of clostridia (174). In addition, these epidemic strains have reduced susceptibility to fluoroquinolone antibiotics compared with both older isolates of the same type and contemporary nonoutbreak strains of C. difficile (130) (see below). This profile has since been assigned North American pulsed-field type 1 (NAP1), restriction endonuclease analysis (REA) group BI, and PCR ribotype 027 (sometimes referred to as BI/NAP1/027). This diverse set of bacterial typing techniques reflects, at present, the lack of a common global currency for C. difficile typing studies, which would help to facilitate a clearer epidemiological understanding of the disease (101). In this review, for simplicity, we have favored the use of the term “ribotype 027” when referring to this strain group.
Two key issues have hindered our understanding of the epidemiology of CDI. First, although there have been improvements in the surveillance of CDI, notably driven by the increased recognition of serious infection and outbreaks, the ascertainment of infection is still variable both within and between the majority of countries. Following early outbreaks caused by C. difficile ribotype 027 in Europe, the European Centre for Disease Prevention and Control (ECDC) convened a group of experts with input from the U.S. Centers for Disease Control and Prevention (CDC). The ECDC working group produced the first background paper on the emergence of CDI in Europe that included interim case definitions for CDI as well as other recommendations for surveillance (109). Very similar recommendations for surveillance were also reported by a CDC working group (132). However, surveillance systems for CDI tended to be initiated only after disease patterns have changed, meaning that there is often poor recognition and documentation of the early phases of changes in epidemiology. Furthermore, as interest in CDI has increased, there is no doubt that ascertainment bias has affected incidence data. Thus, the magnitudes of reported increases in CDI incidence are likely exaggerated, as improved systems to diagnose, enumerate, and analyze cases have been put in place. A good example here is the clear difference in recorded cases of CDI when mandatory data are compared with those provided on a voluntary basis; there were approximately 25% more cases in the mandatory data set from England in 2008 (40,705 versus 32,602) (81, 82). In order to be best placed to control CDI and especially to react to new shifts in disease patterns and presentations, we need to heed the lessons learned during this millennium and optimize surveillance.
A second key impediment to accurate data on epidemiology is the many different approaches to the laboratory diagnosis of CDI (45, 59, 158). Full details of this controversial area are outside the scope of this review. However, it is important to acknowledge that given the different targets and combinations that can be sought when detecting C. difficile (bacterium, glutamate dehydrogenase, toxins, and toxin genes), it is inevitable that the measured incidence of infection may vary according to laboratory method. The most common laboratory test used to diagnosis CDI is an enzyme immunoassay (EIA) to detect C. difficile toxins A and B. It is clear, however, that these tests generally have suboptimal sensitivity and specificity (45, 59, 158).
The surge in interest in C. difficile is typified by a wealth of genome studies (117, 123, 184), including full-genome sequencing, that have recently been reported. For example, the epidemic 027 strain has acquired a complete set of Agr genes compared with earlier strains (184). Also, a C. difficile strain whose tcdB gene was knocked out lost its pathogenicity in hamsters, whereas tcdA knockout strains were as virulent as wild-type bacteria (123). However, before drawing the conclusion that tcdB is the key factor in virulence, this observation needs confirmation, particularly as the mutants generated in this study were inherently unstable. Recently, a research group from Nottingham, United Kingdom, was unable to reproduce this observation (S. Kuehne and N. Minton, personal communication) using a different knockout technique, which generated stable mutations using ClosTron technology (83). Such approaches, especially when married with high-quality epidemiology, will likely provide key information about disease pathogenesis and potential interventions in CDI.
CHANGING C. DIFFICILE EPIDEMIOLOGY IN EUROPE
The first Europe-wide data on the incidence of CDI became available from a survey involving 212 hospitals that was performed by the European Study Group of C. difficile (ESGCD) in the United Kingdom, France, Belgium, Denmark, Germany, Italy, the Netherlands, and Spain in 2002 (13). The incidence of CDI varied considerably, depending partly on the diagnostic testing approaches in use. The mean incidence of CDI was 11 cases per 10,000 admissions. In 2005, the ESGCD performed a second 2-month Europe-wide survey of 38 hospitals in 14 countries (14). All inpatients with CDI were included; a case was defined as a patient with diarrhea and a positive toxinogenic culture. The mean incidence of CDI was 2.45 cases per 10,000 patient days but ranged from 0.13 to 7.1 cases/10,000 patient days. Comparison with the study performed in 2002 was not possible due to differences in design and data analyses. In total, 354 toxinogenic C. difficile isolates were collected and characterized. More than 66 different PCR ribotypes were recognized, and their distribution varied markedly among hospitals and countries. Of all toxinogenic isolates, ribotype 001 was the most common (13%), followed by ribotype 014 (9%); ribotypes 002, 012, 017, 020, and 027 were each found in 6% of toxinogenic isolates, whereas ribotype 078 was found in 3% of toxinogenic isolates. In this survey, ribotype 027 was reported from Ireland, Belgium, and the Netherlands. Patients infected with ribotype 027 were more likely to have more severe disease and to have been treated with metronidazole or vancomycin than patients infected by another PCR ribotype.
In the period between October 2003 and June 2004, the first known large United Kingdom outbreak caused by ribotype 027 occurred at the Stoke Mandeville Hospital (Buckinghamshire Hospitals NHS Trust), involving 174 cases and 19 (11%) deaths that were definitely or probably due to C. difficile (177). A second outbreak occurred between October 2004 and June 2005 in the same hospital, involving 160 new cases and 19 (12%) further deaths. A subsequent United Kingdom Healthcare Commission investigation concluded that the outbreaks were a consequence of a poor environment for patient care, poor practice in the control of infection, lack of facilities to isolate patients, and insufficient priority given to the control of infection by senior managers (44).
In England there is mandatory reporting of all CDI cases for each hospital, and quarterly data have been made publicly available since 2004. There has been a marked decrease in the incidence of CDI, after years of steady increases, since the introduction of enhanced surveillance in 2007 and a centrally funded scheme that provides rapid access to ribotyping (C. difficile Ribotyping Network [CDRN]). In 2008 to 2009 the CDRN processed 4,682 samples, meaning that the ribotypes are known for approximately 1 in 8 to 9 of all CDI cases in England (80). The most commonly identified ribotypes in England were ribotypes 027 (36%), 106 (13%), and 001 (7%). Notably, compared with data from 2007 to 2008, there was a 19% decrease in the prevalence of ribotype 027. Of the eight ribotypes most commonly seen across Europe (16), six were the top eight most prevalent ribotypes in England in 2008 to 2009 (the exceptions being ribotypes 012 and 018); the most prominent ribotype that is seen in England, but rarely elsewhere, is ribotype 106. Multivariate analysis of CDRN data showed that only ribotype 027 was significantly independently associated with mortality (odds ratio [OR] = 1.9; P < 0.001). There was a quadrupling in the numbers of death certificates where C. difficile was mentioned in England and Wales between 2004 and 2007, whereas there was a 29% decrease in 2008 (144). The successful control of ribotype 027 is likely to explain this marked reduction in CDI-related deaths.
In July 2005, C. difficile ribotype 027 was found in an outbreak in a hospital in Harderwijk, Netherlands (108, 199). The outbreak was noticed because of a clustering of severe CDI cases and a rapid increase in incidence (from 4 to 83 cases per 10,000 patient admissions from 2004 to April to July 2005, respectively). The outbreak ended only after the implementation of the restricted use of cephalosporins and a complete ban on fluoroquinolones in addition to general hygienic measures, cohorting of patients in a separate ward, education of staff, and intensified environmental cleaning (50). In September 2005, a ribotype 027 strain was isolated from four patients with CDI in Leper, Belgium, where the incidence had increased from 10 to 33 cases per 10,000 admissions (93). Soon after the first outbreaks in the Netherlands in 2005, a national surveillance program for C. difficile was initiated by the Leiden University Medical Centre (LUMC) and the Centre for Infectious Disease Control of the National Institute for Public Health and the Environment. Also, a prospective, 4-year surveillance study of the incidence of CDI and the distribution of C. difficile ribotypes was started in 14 Dutch hospitals in June 2006. The mean incidence of CDI measured in 14 hospitals remained stable throughout the study period (18 cases per 10,000 admissions in 2007 and 2008). Between April 2005 and June 2009, 2,788 samples were available for ribotyping. A decrease was seen in the prevalence of ribotype 027 after the second half of 2006. In the first half of 2009, the proportion of ribotype 027 isolates among all CDI cases decreased to 3.0%, whereas the proportion of ribotype 001 isolates increased to 27.5%. It was concluded that currently, there is a significant decrease in ribotype 027-associated CDI in the Netherlands (85).
An update on the spread of C. difficile ribotype 027 in Europe was published in 2007 (110). At this point ribotype 027 had affected health care facilities in 11 European Union member states and in Switzerland. The proportions of hospitals where ribotype 027 had been detected varied from 11% (Scotland) to 100% (Ireland), but large differences in surveillance methodology were noted. Interestingly, the Netherlands and France reported a lower attributable mortality rate associated with CDI caused by ribotype 027 (6.3% and 4%, respectively) than had been reported for the United States and Canada (121, 130, 152, 153).
One year later (July 2008), C. difficile ribotype 027 was detected in 16 European countries, causing outbreaks in Belgium, Germany, Finland, France, Ireland, Luxembourg, the Netherlands, Switzerland, and the United Kingdom (111). Ribotype 027 was also reported on a smaller scale in Austria, Denmark, Sweden, Norway (90), Hungary (191), Poland (157), and Spain, although Austria and Denmark later reported outbreaks of CDI (8, 89). The proportion of affected hospitals in each country varied considerably (0 to 76%), but systematic comprehensive surveillance was performed only in England (110/145 hospitals [76%]), Wales (10/16 hospitals [63%]), and Belgium (32/74 hospitals [43%]). Three countries reported the importation of patients with CDI caused by ribotype 027. In Switzerland, an outbreak of C. difficile ribotype 027 was observed in a geriatric hospital in Basel in 2006 (64). The index case was an 82-year-old female patient who returned from a hospitalization during her holidays abroad. The outbreak involved 15 other patients between October 2006 and May 2007. In 2007 in Spain, a patient who was transferred from a hospital in the United Kingdom was admitted with a severe form of CDI at a large tertiary-care hospital in Madrid (111). Although no spread was found among patients in the hospital, a laboratory technician working with this C. difficile isolate developed CDI shortly after receiving antibiotic treatment (25). In Austria, C. difficile ribotype 027 was reported in 2006 in a British tourist suffering from pseudomembranous colitis who was treated with antibiotics shortly before traveling to Austria (88).
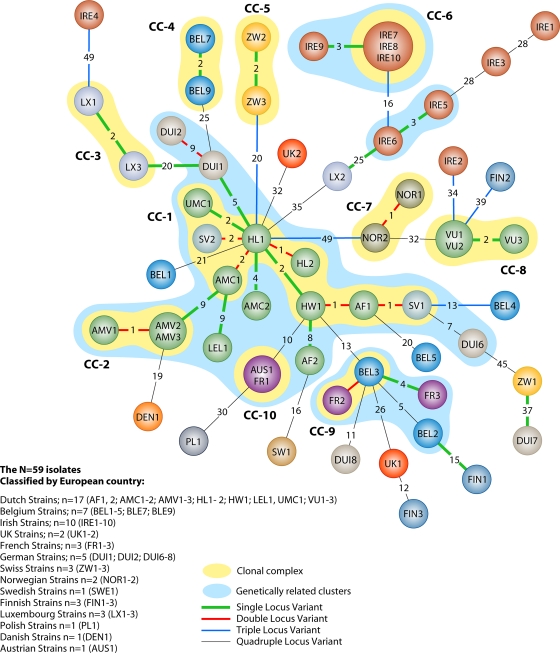
A new finding was the occurrence of outbreaks due to clindamycin-resistant ribotype 027 isolates in Switzerland (64), Ireland (54), France, and, more recently, Denmark (8). Clindamycin resistance was defined as ermB-positive isolates with a clindamycin MIC of >256 mg/liter. Until this finding, the use of clindamycin was relatively “protective” with regard to the development of CDI due to ribotype 027 (76). The emergence of resistance to clindamycin in ribotype 027 isolates may increase the risk of CDI in patients receiving this agent, and its use may be an important factor contributing to strain persistence and spread. Unpublished observations from the National Reference Laboratory at Leiden University Medical Center indicated that clindamycin-resistant ribotype 027 isolates from these three countries were unrelated to each other, as determined by multilocus variable-number tandem-repeat analysis (MLVA) (Fig. 1). In addition, reports of erythromycin-susceptible and clindamycin-susceptible ribotype 027 isolates in Germany and Denmark indicated that antimicrobial resistance to macrolides, lincosamides, and fluoroquinolones varies for C. difficile ribotype 027 isolates.
FIG. 1.
Minimum-spanning-tree analysis of 59 C. difficile ribotype 027 isolates from 27 different regions of 14 countries typed by MLVA. Each circle (colored coded by country of origin [see key]) represents either a unique isolate or more isolates that are 100% homologous. The Dutch strains (green circles) (n = 17) are labeled by the submitting hospital plus a number. The other isolates are labeled by country and a number. The numbers between the circles represent the STRDs between MLVA types. The different-colored lines between the circles are indicative of one (green), two (red), three (blue), or four (gray) locus variants. Clindamycin-resistant isolates (n = 9) are IRE1, IRE5, IRE6, IRE8, IRE9, IRE10, FR1, BEL4, and BEL5. (Courtesy of National Reference Laboratory, Leiden, Netherlands; created by Celine Harmanus and used with permission.)
Recent reports of C. difficile ribotype 027 infection in Finland are of special interest, notably as this country has always been considered a country with a very low incidence of CDI, and comprehensive data mapping the increased incidence of CDI are now available. A first case of fatal C. difficile ribotype 027-associated disease was detected in October 2007 (124). Neither the index case nor two additional cases had connections with foreign countries or each other. Since then, the National Public Health Institute intensified the surveillance and control of CDI. Other ribotype 027 isolates were detected retrospectively, indicating that this strain had previously been circulating in Finland. To determine whether the rate of CDI and related deaths was increasing in Finland, registry data from 1996 through 2004 were investigated (125). Those discharged with a CDI diagnosis doubled from 810 patients (16 patients/100,000 population) in 1996 to 1,787 patients (34 patients/100,000 population) in 2004. The increase was most prominent for patients aged >64 years, and the age-standardized mortality rate associated with CDI increased from 9 per million in 1998 to 17 per million in 2004. In January 2008, a new surveillance module for CDI started as a part of the Finnish Hospital Infection Program (SIRO). A total of 740 cases of CDI were reported from 12 hospitals. The overall rate, nosocomial rate, and prevalence at admission were 0.71 per 1,000 patient days, 0.49 per 1,000 patient days, and 0.71 per 1,000 admissions, respectively. Of the 12 hospitals with reported cases of CDI, 8 had sent isolates for genotyping. Strains of PCR ribotype 027 were detected in three of the eight hospitals (126).
In order to determine the epidemiology of CDI across Europe, the ECDC commissioned a prospective incidence survey of cases in one to seven hospitals per country (according to population size) during November 2008 (16). The study aimed to determine the baseline incidence of hospital- and community-acquired CDI, causative ribotypes, risk factors, and outcomes and to establish a network of 106 laboratories capable of isolating C. difficile strains in 34 European countries. The CDI incidence varied across hospitals (mean, 5.5 cases per 10,000 patient days; range, 0 to 36.3) and countries (range, 0 to 19.1). Sixty-four different C. difficile PCR ribotypes were found, among which ribotypes 014/020 (15%), 001 (10%), and 078 (8%) were most prevalent; the prevalence of ribotype 027 was 5%. Most patients had the classical risk profile of an elderly patient with comorbidity and recent antibiotic use. At follow-up, 22% of patients had died, and for 40% of these cases, CDI was deemed to have played a role. It was concluded that the incidence of health care-associated CDI varied widely between hospitals and countries and was generally higher than documented in 2005.
Highly discriminatory fingerprinting techniques, including restriction endonuclease analysis and MLVA, can distinguish among individual ribotypes, including ribotype 027 strains from different outbreaks (63, 101, 198). For example, MLVA distinguished 23 types within 91 isolates of C. difficile ribotype 027, providing hitherto unrecognized information about related clusters of hospital cases (63). MLVA of 59 C. difficile ribotype 027 isolates from 27 different regions of 14 different European countries is shown in Fig. 1. To study phylogeny, minimum-spanning-tree (MST) analysis of MLVA types was performed by using the number of differing loci and the summed tandem-repeat difference (STRD) as coefficients for the genetic distance. Isolates with an STRD of ≤10 were defined as genetically related, and clonal complexes were defined by an STRD of ≤2. Of seven loci used for MLVA, two did not show variation in any of the isolates, three showed a large variation, and two remained “intermediate”-variability loci. In total, the 59 isolates clustered into 54 unique MLVA types. Four types contained more than one isolate, which were 100% identical: three types contained two isolates, and one type contained three isolates. Among the MLVA types, four genetically related clusters (GCs) and 10 clonal complexes (CCs) were recognized. The largest GC contained isolates from four different countries, two GCs were country specific (Ireland), and one GC contained isolates from Belgium and France only. Among the 10 CCs, 8 were country specific (CC-1 through CC-8), whereas two clonal complexes contained isolates from two different countries (CC-9 and CC-10). In one country, three clonal complexes (CC-1, CC-2, and CC-8) were identified. Two of these clonal complexes were region specific (CC-2 and CC-8). Of the nine clindamycin-resistant ribotype 027 strains, six (67%) originated from Ireland, and five of these strains clustered into two GCs. The four clindamycin-resistant strains that did not show clustering originated from three countries (two from Belgium and one each from Ireland and France). This analysis demonstrates the power of highly discriminatory fingerprinting techniques to provide information on isolate relatedness.
CHANGING C. DIFFICILE EPIDEMIOLOGY IN NORTH AMERICA
There have been well-documented changes in the incidence and severity of CDI across Canada and the United States over the last decade. A retrospective study of all cases of CDI over a 13-year period in Quebec, Canada, reported an increase in incidence from 35.6 cases per 100,000 population in 1991 to 156.3 cases per 100,000 population in 2003 (152). The proportion of infections that were complicated increased from 7.1% to 18.2% in this period, and the 30-day mortality rate increased from 4.7% to 13.8%. A later study established similar rates across the entire Sherbrooke, Canada, and Montreal, Canada, regions. The reported incidence was 22.5 cases per 1,000 admissions, which was associated with an unexpectedly high attributable mortality rate of 6.9% (121). In the United States, concurrent studies in Pittsburg, PA, established a similar epidemiological picture and reported increased incidence (2.7 cases per 1,000 discharges in 1997 to 6.8 cases per 1,000 discharges in 2001) and severity (0.15 to 0.60 cases per 1,000 discharges) of CDI (46, 139). More recently, U.S. studies have continued to report increases in CDI incidence and severity in specific institutions or states (37, 98, 190). Population-based data studies have strengthened these reports. The National Hospital Discharge Survey, representing 500 hospitals across the United States, revealed that the number of hospital discharges where CDI was listed had doubled between 1996 and 2003 (131). A 20% subset of data from the Nationwide Inpatient Sample also concluded that the incidence, case fatality rate, total mortality rate, and colectomy rate for CDI all significantly increased over the same period (159).
The increase in numbers of CDI in Canada and the United States has been attributed largely to the spread of the BI/NAP1/027 (ribotype 027) strain. Indeed, this type has now been found in all Canadian provinces and in at least 40 states in the United States (143). In Canada, the highest incidence of severe CDI and ribotype 027-associated infection has remained focused in Quebec. A 6-month prospective surveillance study (November 2004 to April 2005) covering nine Canadian provinces recently reported an overall CDI rate of 4.6 cases per 1,000 patient admissions but with a higher rate for hospitals in Quebec than for those in the rest of Canada. Additionally, CDI-attributable mortality remained more than four times higher in Quebec than that for the rest of Canada combined. A subsequent molecular analysis of a subset of C. difficile isolates demonstrated the presence of ribotype 027 in all Canadian provinces studied but most frequently in Quebec, Ontario, and British Columbia (79). In a study representing three different Canadian health regions from 2001 to 2004, ribotype 027 represented 75.2% of hospital isolates from the Montreal area (absent in 2000 and 2001), compared with 7.4% and 5.9% in Alberta and British Columbia, respectively. Further analysis of a subset of ribotype 027 isolates established a predominant subtype common to all three regions (127).
The evidence that ribotype 027 has been solely responsible for the increased incidence is not definitive. Studies have conflicted when comparing outcome data for patients with CDI due to ribotype 027 strains with data from those infected with other C. difficile types. A definitive Canadian study showed that the 30-day mortality rate for patients with ribotype 027 CDI was twice that for those infected with a toxinotype 0/ribotype 001 strain (114). Conversely, Chopra et al. recently reported no significant differences in risk factors, severity of disease, and outcome in a comparison of infections caused by BI/NAP1/027 and non-ribotype 027 strains (41). One study found that 30-day mortality was twice as likely in CDI caused by ribotype 027 but that this difference was not significant when the data were controlled for age (87). Confounding factors such as unrecognized independent risk factors, temporal changes in patient mix, and infection control practices make studies of this nature very hard to compare with confidence.
Progress in the understanding of CDI epidemiology in the United States in recent years has been slowed by the lack of standardized surveillance methods and reporting definitions. As a consequence of the increased or mandatory surveillance of CDI, guidelines and CDI case definitions have been reexamined. A study in Ohio reported that the definitions for health care-associated and community-associated CDI drawn up by the Ohio Department of Health in 2006 were discordant with those formulated locally and agreed in only 71% of cases (65). An ad hoc C. difficile surveillance working group reported recommended definitions for (i) health care- and community-associated disease, (ii) severity of disease, and (iii) standardized methods for reporting (132). These definitions matched those reported previously for Europe (109). Subsequent surveillance definition studies have helped to consolidate these recommendations, and they have become widely adopted (58, 112). U.S. studies have continued to report increased CDI rates (33), and projected estimates for CDI in the U.S. by 2010 have reached 450,000 to 750,000 cases per annum (134, 135, 219). A recent analysis detected a 23% annual increase in CDI-related hospitalizations between 2000 and 2005, associated with an increased age-adjusted, annual case-fatality rate of 0.2% over the study period (218). However, a subsequent extension of that study reported a reduced rate of increase of CDI-associated hospitalizations in the following year (only a 6.7% rise in cases for 2005 to 2006). This was associated with a reduction in CDI-associated hospitalizations in the northeastern United States and other U.S. states (220). Decreased rates of infection associated with those states originally at the epicenter of the outbreak may signal a turning point in the pattern of spread in the United States.
Recent U.S. studies have suggested that the epidemiology of CDI in patients thought previously to be of low risk is also changing. Increased rates of infection in several peripartum women and patients from the community setting have been recorded in multiple states (35) (see below). The true extent of CDI in children remains debatable due to the increased potential for the asymptomatic carriage of C. difficile in this patient group (116). However, U.S. studies have recently reported increased rates of infection in this cohort, although such reports should be treated with caution because of potential ascertainment bias secondary to increased or method changes in laboratory testing. Klein et al. reported an unexpectedly high C. difficile culture rate (26%) for children who presented with diarrhea via a hospital emergency room between 1998 and 2001 (105). In Washington, the proportion of children with toxin-positive stool tests increased from 46% in 2001 to 64% in 2006. In that same study, severe gastrointestinal disease was seen in 91% of neonates with CDI in the intensive-care setting (21). An analysis of data from 22 hospitals across the United States reported marked increases in pediatric cases of CDI but with no increase in severity of disease or complications (103). A more recent study of all U.S. pediatric hospitalizations due to CDI (which were included in the National Hospital Discharge Survey between 1997 and 2006) reported a rate increase of 7.24 to 12.80 cases per 10,000 discharges. Incidences in newborns, nonnewborns, 1- to 4-year-old patients, and 5- to 9-year-old patients were 0.5, 32.01, 44.87, and 35.27 cases per 10,000 discharges, respectively. More studies are clearly needed to understand the true significance of C. difficile in children.
Data on the epidemiology of U.S. endemic and outbreak C. difficile strains in the last few years are fragmented. Many reports have either not included typing data at all or analyzed only a subset of implicated strains, often from only their own institutions. For example, in an investigation of an outbreak potentially associated with a switch to moxifloxacin use, only 12% (6/50) of strains were characterized. Equal numbers of toxinotype III (ribotype 027-associated) and toxinotype 0 strains were identified (23). Toxinotype 0 strains have continued to represent significant proportions of non-ribotype 027 type strains implicated in disease. This most likely represents continuing endemicity from outbreaks associated with REA type J in the 1990s (92). According to more recent studies where adequate molecular characterization has been performed, ribotype 027 is now firmly associated with hospital-associated CDI in nonoutbreak settings. A study in Michigan recently examined 30 C. difficile isolates from symptomatic stem cell transplant recipients and patients with and without cancer. Ribotype 027 was found in 33% patients overall. Seven different pulsotypes were found among the remaining isolates in that study (41). A recent study used REA to characterize a collection of 548 C. difficile isolates representing CDI cases from 45 locations in 25 U.S. states between 2006 and 2009. REA group BI (ribotype 027) represented 54% of the collection and was identified in 80% of U.S. states and 82% of individual hospitals; the isolates were submitted voluntarily and, thus, could be biased toward more severe CDI. Ribotype 027 strains were further divided into five REA subtypes, three of which (subtypes 6, 8, and 17) predominated. The next most commonly identified groups (groups Y [8%] and J [4%]) were considerably less common (38). The proportion of ribotype 027 isolates in this statewide collection is comparable to that recorded (51%) in an earlier report of a U.S. ribotype 027 outbreak in 2000 to 2003 (130). Clearly, ribotype 027 has persisted as the dominant C. difficile epidemic strain in the United States, although the lack of systematically collected data obscures our understanding of real-time changes and the success of control measures. Interestingly, it was recently reported that ribotype 106, hitherto known primarily as a United Kingdom epidemic strain, has been detected in North American isolates collected in 2008 to 2009; however, subtyping using REA demonstrated no overlap with strains from Europe (171).
CHANGING CDI EPIDEMIOLOGY IN THE REST OF THE WORLD
As interest in CDI has increased, we have a better appreciation of the differences in ribotype distributions in some countries. The travel and transport of patients with CDI may introduce new PCR ribotypes that can replace other types or cause new outbreaks. The first patient in Australia with CDI due to ribotype 027 was a 43-year-old woman with a permanent ileostomy who appears to have been infected while traveling in the United States (164). Unfortunately, few data were available from Australia on the incidence of CDI and the distribution of the various ribotypes before the introduction of ribotype 027. Although the incidence was high during the 1980s in hospitals in Western Australia, a significant decrease was observed, from 2.09 cases per 1,000 discharges in 1998 to 0.87 cases per 1,000 discharges in 1999 (P < 0.0001); this decrease persisted into 2000 and was assumed to correlate with a decreased use of broad-spectrum cephalosporins (193).
C. difficile ribotype 027 has also been detected in Japan. The first reported patient was a 30-year-old Japanese woman hospitalized for ulcerative colitis who developed pseudomembranous colitis 1 month after admission (97). In contrast to ribotype 027 strains circulating in North America and Europe, this isolate was susceptible to the newer fluoroquinolones, as was reported for those strains obtained before 2001 in North America. The epidemiological changes in PCR ribotypes in Japan are illustrated by the results of a 5-year study in a teaching hospital, where a shift was observed from predominant ribotype a in 2000 (15/33 cases [45%]) to ribotype f in 2004 (18/28 cases [64%]). Ribotype f is identical to the PCR ribotype designated “smz,” which was reported to have caused multiple outbreaks in Japan and is suspected to correspond with ribotype 001 (173). This shift was not associated with clear changes in the clinical characteristics of CDI. Another interesting finding was that of the 148 isolates examined, 33 (22%) were TcdA negative, TcdB positive, and cytotoxin negative. Most toxin A-negative, toxin B-positive (A− B+) C. difficile isolates belong to ribotype 017 (196), which is found more frequently in Asia than in some other continents. The clinical manifestations of and risk factors for CDI caused by ribotype 017 were retrospectively investigated in a small study in a Japanese hospital (106). Three factors were found to be associated with infection by A− B+ isolates: exposure to antineoplastic agents, the use of nasal feeding tubes, and care in a particular medical ward. There was no statistically significant difference in temperature, serum C-reactive protein (CRP) levels, white cell count, frequency of diarrhea, or type of underlying disease in cases compared with toxin A-positive toxin B-positive (A+ B+) patients (106).
A recent shift in the distribution of various C. difficile PCR ribotypes has also been observed in South Korea. Although the prevalence of A− B+ C. difficile strains in six hospitals was <5% before 2000, it began to increase in 2003 (13%) and peaked in 2004 (50%). In 2005, the mean prevalences of A+ B+ and A− B+ strains were 48% (31 to 60%) and 27% (11 to 55%), respectively (175). Among 29 cases of pseudomembranous colitis, 21 (72%) were associated with A− B+ strains; the authors of that study concluded that these variant strains could evoke a higher rate of PMC than A+ B+ strains (176), but this observation has not been confirmed by others. Kim et al. reported a similar increased prevalence pf A− B+ strains in South Korea (102). The first case of CDI due to ribotype 027 in South Korea was recently described (188).
Since the first reports on emerging CDI associated with ribotype 027 appeared in the literature, several laboratories and hospitals in China initiated surveillance studies. In a collection of 75 clinical isolates from Shanghai, China, 33% were ribotype 017 A− B+ strains, but no ribotype 027 strains were present (86). The detection of C. difficile ribotype 027 in Hong Kong was recently reported for a case of locally acquired health care-associated CDI; no secondary cases occurred (39). From June to December 2008, the Chinese Center for Disease Control performed a survey among hospitalized patients who were older than 18 years and who developed diarrhea. Of 112 fecal samples, 12 (11%) contained C. difficile, 8 of which were toxinogenic, including 3 A− B+ strains, but ribotype 027 was not found (40; E. Kuijper, personal communication).
Only a few reports of CDI in Latin America are available. A recent literature review using medical databases of Latin American countries identified seven recent papers in which clinical characteristics and risk factors were analyzed (32). The attributable mortality rate was lower than that reported for hospitals in other developed countries, but there is clearly a need for better prospective studies to determine the epidemiology of CDI in more detail. In a recent study in Brazil, C. difficile strains from fecal and hospital environmental samples were characterized. Although ribotype 027 was not reported, strains belonging to ribotype 106 were detected; this appears to be the first report of this ribotype outside the United Kingdom (11). A recent survey of a 200-bed Argentinean general hospital revealed CDI incidence rates of 37 to 84 cases per 10,000 admissions between 2000 and 2005 (78). The increased incidence prompted the initiation of systematic surveillance, which showed a high prevalence of ribotype 017. Interestingly, when multiple-locus variable-number tandem-repeat analysis (MLVA) was applied to 56 Argentinean isolates and 15 isolates from seven other countries, country-specific clonal complexes were found. This finding was in contrast with data from a previous report in which 39 A− B+ isolates from seven countries were investigated by use of amplified fragment length polymorphism (AFLP) and two PCR ribotyping methods. It was concluded that clindamycin-resistant ribotype 017 had a clonal worldwide spread, but this conclusion was not supported by data obtained by using the highly discriminatory MLVA technique (197).
No recent information on CDI prevalence rates or microbiological characteristics of C. difficile strains isolated from humans is available for Africa. CDI data from Middle East countries are sparse. C. difficile isolates (n = 113) from patients and the environment of intensive therapy units (ITUs) of four teaching hospitals in Kuwait were of 32 different ribotypes. The predominant ribotypes of clinical isolates were ribotypes 097 and 078, and eight strains did not match any strains in the PCR ribotype library established at the Anaerobe Reference Unit, Cardiff, United Kingdom (167). A recent prospective study among hospitalized adult Jordanian patients did not provide data on CDI incidence or strain characteristics (141).
COMMUNITY-ACQUIRED CDI
Although C. difficile is a ubiquitous microorganism that may be found in the environment, animals, and food, its prevalence is thought to be so much higher in health care facilities that, for a long time, the acquisition of CDI was thought to occur almost exclusively during or shortly after admission to such establishments. The high incidence of CDI in health care facilities compared with the community presumably results from the high density of individuals prone to CDI, classically, elderly patients with comorbidity, who may serve as a reservoir in which C. difficile can amplify. However, it is increasingly being recognized that some CDI cases are acquired outside health care facilities. Several studies have shown various proportions of CDI cases to be “community acquired,” but crucially, the definitions of community acquisition differ. Some studies did not systematically examine previous admissions to health care facilities (120, 161, 162, 163, 210). Others variably defined an “exclusion” period to distinguish patients recently admitted to a health care facility.
An ad hoc C. difficile surveillance working group (109, 132) advocated a division of CDI into health care-associated and community-associated CDI. The latter is defined as CDI with onset in the community (or in a health care facility within 48 h after admission) for a patient who has not been discharged from a health care facility in the previous 12 weeks. If the patient was discharged 4 to 12 weeks previously, such a case should be classified as having an indeterminate association. A further difficulty concerning studies of community-acquired CDI is patient selection. The studied patient population may strongly influence the extrapolated incidence in the community. An estimation of incidences may also be hampered by the uncertainty of the denominator size, i.e., the population from which stool samples were collected (for example, a laboratory's community catchment area). The issue of the important effect of diagnostic methods on reported CDI epidemiology is discussed in the Introduction. Poor assay specificity will result in overestimates of CDI cases, particularly in low-incidence settings such as the community. This may be compounded if other enteropathogens are not ruled out systematically as potential causes of community-associated diarrhea.
In 1995, Karlström et al. (96) conducted a nationwide survey of CDI in Sweden. Among 1,888 CDI cases, defined as unique patients with toxin-positive fecal samples from 13 of Sweden's 31 microbiological laboratories, 28% were reported as community acquired, defined as community onset without hospitalization in the preceding 4 weeks. Patients with community-acquired CDI were younger than patients with health care-associated CDI. A study in Ireland reported that 11% of 73 new CDI cases (diarrhea with positive stool cytotoxicity test) were community acquired, defined as occurring upon or within 72 h of admission without hospitalization in the previous 60 days (113). In 1999, a Swedish study (142) investigating all cytotoxin-positive diarrheal stool samples submitted to three major county hospitals found 22% of 267 cases of first episodes of CDI to be community acquired (without hospitalization in the previous 60 days). Again, patients with community-acquired CDI were younger than their counterparts with nosocomial CDI. Similar findings were confirmed by another Swedish study (187). A hospital-based study conducted in 2005 in the Netherlands (147) found 20% of 81 CDI cases (diarrhea and toxin-positive stool) to be cases of community-onset (without hospitalization in the preceding 4 weeks) CDI. After the implementation of a statewide surveillance system in Connecticut in 2006, 60% of 400 evaluable community-onset CDI cases (gastrointestinal symptoms and a positive toxin assay) reported that year by acute-care hospitals met the definition of community acquisition (no admissions to health care facilities in the previous 3 months) (36). A recent European surveillance study of 29 European countries used the above-described C. difficile surveillance working group definitions and found that the ratio of community-associated CDI and health care-associated CDI varied among participating hospitals, although it was mostly low, with only 8/83 hospitals reporting more community- than health care-associated cases (17).
A study based on data from the United Kingdom General Practice Research Database (52) evaluated the incidence of community-acquired CDI (defined as patients with toxin-positive feces or a clinical diagnosis of CDI who had not been hospitalized the previous year, divided by the number of inhabitants registered with general practitioners providing data for this database). The incidence increased from 0 to 18 cases per 100,000 persons per year between 1994 and 2004. Dial et al. (52) found that inflammatory bowel disease (relative risk [RR], 4.1; 95% confidence interval [CI], 2.6 to 6.6), irritable bowel syndrome (RR, 3.4; 95% CI, 2.3 to 5.0), hospitalizations in the previous 2 years (RR, 2.2; 95% CI, 2.0 to 2.4), and renal failure (RR, 1.7; 95% CI, 1.2 to 2.2), and the use of proton pump inhibitors (PPI) (RR, 1.6; 95% CI, 1.3 to 2.0), but not histaminic receptor-blocking gastric acid suppressants, were significantly associated with CDI. Wilcox et al. estimated the incidences of community-associated CDI in 2000 in two distinct settings in England, one urban (29.5 cases per 100,000 population) and the other rural (20.2 cases per 100,000 population), by examining fecal samples (n = 2,000) submitted by general practitioners from individuals with diarrhea in the community (214). In both settings, 2.1% of samples were cytotoxin positive. Exposure to antibiotics in the previous 4 weeks, particularly multiple agents (P < 0.001), aminopenicillins (P < 0.05), and oral cephalosporins (P < 0.05), was significantly more frequent among CDI cases than among age- and sex-matched cytotoxin-negative diarrheal controls from the same general practices. Notably, hospitalization in the preceding 6 months was significantly associated with CDI (45% versus 23%; P = 0.02). However, almost half of the cases had not received antibiotic therapy in the previous month, and approximately one-third had neither exposure to antibiotics nor recent hospitalization. Interestingly, contact with infants aged <2 years was significantly associated with CDI (14% versus 2%; P = 0.02). Weil and coworkers (206) also examined fecal samples for evidence of CDI irrespective of the requested diagnostic test but used a toxin EIA (and therefore, the specificity of the results is questionable). Those researchers found that 9.2% of 703 stool samples from individual patients were EIA positive; 53% of these patients had not been hospitalized in the previous 4 weeks. A recent large study in the Netherlands (17) examined all fecal samples submitted to three regional microbiological laboratories by using a toxin EIA. The proportion of positive fecal samples (1.5% of 2,443 samples) was remarkably similar to that seen in the English study (214). Among those patients with toxin-positive stool samples for whom information was available, 20 (65%) had not been admitted to a health care institution in the previous year, 13 (42%) had not used antibiotics during the 6 months previous, and 8 (26%) had neither risk factor. In the English study, the most common bacterial enteropathogens were ruled out, while the Dutch study examined only those pathogens originally requested by the general practitioner. Thus, these studies demonstrate a striking difference in studies on nosocomial CDI, which usually show that the great majority of patients have received antibiotics. The studies allow more reliable estimates of community-associated CDI to be made, but selection bias may remain. For instance, it is possible that only more prolonged or severe cases led to sample submission. Evidence of this phenomenon comes from a French study (19) in which ambulatory patients who were prescribed an antibiotic by their general practitioner for the first time in at least 2 months and who had not been hospitalized in the previous 6 months were monitored prospectively to examine the development of CDI. Of 260 evaluable patients, 1 was an asymptomatic carrier at baseline and remained so during 14 days of follow-up; 7 patients acquired a toxigenic C. difficile strain, and 4 of these patients developed diarrhea, which was self-limiting in all cases.
If the incidence of community-acquired CDI is indeed increasing and rising awareness does not account for this increase, then what could be the source of exposure in the community? Of course, it can be argued that increased pressure from hospitals could propagate the increased circulation of strains in the community. However, in the Dutch study, 29 isolates underwent PCR ribotyping, revealing many uncommon ribotypes, and notably, ribotype 027 was not found (despite this ribotype having recently caused major nosocomial outbreaks in all regions investigated), which might argue against increased pressure from hospitals (17). In contrast, two earlier Swedish studies (142, 187) found similar distributions of PCR ribotypes among nosocomial and community-acquired cases. Animals are obvious candidates for a community reservoir, since C. difficile may be a commensal and a pathogen in animals, and farm animals are often exposed to antibiotics. Spores might be spread either by direct contact with animals and their feces or by the consumption of contaminated meat. Several studies, which will be discussed below, have demonstrated the presence of C. difficile in animal feces and meat. Evidence for farm animals as a potential reservoir for C. difficile may come from epidemiological associations and typing studies. In the Dutch study (17), the proportion of positive stool samples was highest in more rural communities, but here and in an earlier Australian study (162), there were no patients who reported contact with farm animals. However, in a more rural part of the Netherlands, 4 out of 10 CDI cases in which ribotyping was performed were caused by ribotype 078, whereas this ribotype was found in neither of the other regions. This ribotype is frequently found in farm animals and has been associated with community-associated CDI in another Dutch study (77). Multilocus variable-number tandem-repeat analysis of ribotype 078 isolates in that study revealed four clonal complexes to which both porcine and human strains belonged. Epidemiological data on C. difficile in animals and food is discussed in more detail below (see New Information on Etiology of and Risk Factors for CDI).
NEW INFORMATION ON ETIOLOGY OF AND RISK FACTORS FOR CDI
Gastric Acid Suppressants
The use of gastric acid suppressants, especially PPI, has been proposed as a risk factor for acquiring CDI. Theoretically, these PPI could decrease the colonization barrier against vegetative forms of C. difficile by elevating the gastric pH. However, C. difficile spores are resistant to gastric acid, and it is likely that spores represent the main mode of acquisition. A large number of studies have associated the use of gastric acid suppressants with the acquisition of CDI. A systematic review (119) found the use of H2 receptor antagonists (H2RA) and PPI to be significantly associated with having CDI (pooled ORs, 1.48 [95% CI, 1.06 to 2.06] and 2.05 [95% CI, 1.47 to 2.85], respectively), but the included studies were very heterogeneous. Notably, most studies have investigated such associations in the setting of a CDI epidemic in the hospital, which may not be a reliable setting given the potential for confounding variables. Two studies examined the association of PPI with CDI in a hospital setting where the disease is endemic. Dalton and colleagues (47) found several variables, including 10 drug classes apart from antibiotics, to be associated with the development of CDI in a univariate analysis. A logistic regression model that included the use of antidepressants, number of medicines used, days of antibiotic use, age of patient, length of hospital stay, and admission to a medical ward versus a surgical ward was constructed. The odds of CDI increased by 1.01 (95% CI, 1.00 to 1.02) for each day of PPI use; H2RA use increased the odds by 1.7 (95% CI, 1.09 to 2.64). A study by Dubberke et al. (55) found significant ORs of 1.6 and 2.0 for PPI and H2RA, respectively, using a logistic regression model that incorporated previous admissions, age of patient, hematological malignancy, albumin level, mechanical ventilation, several antibiotics, and “C. difficile-associated diarrhea (CDAD) pressure” (exposure to other CDI cases), a variable that has been the subject of some debate and will be discussed elsewhere. Concerning community-acquired CDI, PPI, but not H2RA, was found to be a risk factor in one study, as discussed previously (53), but this was not found by two other studies of patients with community-acquired CDI (122, 214). Some studies have examined the prognostic significance of gastric acid suppression in CDI. H2RA have been associated with a higher chance of recurrence (189), and PPI have been associated with refractory CDI (1). However, a recent study found no association between either group of gastric acid suppressants and a more severe course of CDI (84).
In spite of the extent of the literature, it is still a matter of ongoing debate whether there is a causal association between PPI or H2RA and CDI. The main reason for this is the fact that gastric suppressants may be given frequently to patients with certain comorbidities as a prophylaxis (e.g., those who use anticoagulants or nonsteroidal anti-inflammatory drugs [NSAIDs] and severely ill patients in intensive-care units [ICUs]). These factors may themselves be risk factors for or associated with other risk factors for CDI. Hence, it may be extremely difficult, if not impossible, to correct for these confounders in case-control studies. The only definitive answer might come from a randomized controlled trial that systematically evaluates both colonization with C. difficile and the development of CDI in patients taking gastric acid suppressants.
Animals and Foods as Potential Sources of C. difficile
C. difficile is ubiquitous in the environment. In a large and classical study of the distribution of C. difficile in the environment of South Wales, United Kingdom, a total of 2,580 samples were taken, with 184 (7.1%) yielding isolates (2). The highest yield of C. difficile was obtained from river (87.5%) and seawater (44%) samples, but it was also isolated from swimming pools (50%) and main tap water (5.5%). In private residences, the organism was present in 12 (2.2%) of 550 samples, whereas 2.4% of 300 raw-vegetable samples were positive. The rate of carriage of C. difficile in 524 fecal samples from assorted farm animals was 1%, while rates were 10% in dogs and 2% in cats. C. difficile has also been found in calves, ostriches, chickens, elephants, dogs, horses, and pigs, but its role in infection and its pathogenesis in animals are largely poorly understood and possibly underestimated (168). Similarly, any association between antibiotic usage and C. difficile colonization or diarrhea in animals is less well documented than that in humans, although the acquisition of C. difficile in dogs and cats during hospitalization in an ICU was associated with the development of diarrhea (43). In an experimental study, it was also demonstrated that erythromycin can induce severe colitis associated with the proliferation of C. difficile in mature horses (18).
Several C. difficile ribotypes have been found in animals, although the heterogenicity appears to be much less than that found for human CDI (168). Several indistinguishable strains may be found in multiple species (6), which suggests that there is a potential for the interspecies transmission of C. difficile. C. difficile ribotype 027 is not an emerging pathogen of disease in animals, since no documented outbreaks have been reported, and only occasional isolates have been described (181). However, C. difficile is known as an important pathogen of horses, and it has been reported to infect piglets and calves. In a Canadian study of calves from 102 dairy farms, fecal samples from 144 and 134 diarrhea and control animals, respectively, were cultured for C. difficile and tested by using an EIA for C. difficile toxins A and B. Toxins were detected in 40% of calves with diarrhea but also in 21% of healthy controls. Eight different ribotypes were found, seven of which have been shown to cause CDI in humans, including outbreaks of severe disease (ribotypes 017 and 027) (165). A search for C. difficile in various animals in the United Kingdom examined neonatal pigs, male dairy calves (<2 months of age), horses, and dogs. The 232 isolates obtained comprised 19 ribotypes, with the predominant one being ribotype 078 in calves and pigs (99). Recently, two herds of piglets suffering from diarrhea in the Netherlands proved to be excreting C. difficile toxinotype V, PCR ribotype 078 (49). Thirty-one C. difficile isolates were obtained from 48 animals. C. difficile was not detected in mother sows or in 272 healthy, weaned piglets on seven farms. None of the farm workers and none of the family members of the farm owners had CDI. C. difficile ribotype 078 isolates from humans and pigs were highly genetically related; as shown by MLVA, all the isolates contained a 39-bp tcdC deletion and a point mutation at position 184, and most produced binary toxin. These findings suggest a common source and/or that recycling is common. Jhung et al. (91) also found that food animal and human isolates had a high degree of similarity, as determined by REA and pulsed-field gel electrophoresis (PFGE) subtyping. While ribotype 078 appears to be a pathogen in some piglets, a study of C. difficile in Spain including 780 animals from 13 pig farms found that 26% of newborn piglets but no 1- to 2-month-old piglets were C. difficile positive, irrespective of the presence of diarrhea (4). Similar findings were reported for piglet cohorts in a recent study in England, where despite the frequent presence of C. difficile ribotype 078, no animals had overt evidence of infection (L. Brunton, J. S. Knapp, J. Heritage, S. D. Baines, J. Freeman, W. N. Fawley, M. H. Wilcox, and H. M. Miller, submitted for publication). A recent study performed in Canada evaluated C. difficile colonization in dogs and cats in a longitudinal manner and found that C. difficile is commonly, albeit sporadically, found in the feces of healthy pets. C. difficile was isolated from 14/139 (10%) dogs and 3/14 (21%) cats. Thirteen (93%) of the dogs and all of the cats that tested positive were positive only by one of the five samples. All toxigenic strains identified in pets have been isolated from humans in Ontario, Canada. Although the prevalence of C. difficile in dogs and cats is low, the fact that all toxigenic strains are recognized human pathogens raises concern about interspecies transmission (205).
Spores of C. difficile can also be detected in meat products and ready-to-eat salads (10, 182, 201, 204), but there is no conclusive evidence that C. difficile contamination of food has led to clinical CDI in humans. Additionally, no food-borne disease outbreaks of C. difficile have been reported. In a recent Canadian study, C. difficile was isolated from 14/115 (12%) ground-beef samples and 14/115 (12%) ground-pork samples (204). Ribotype 078 predominated, consistent with data from some previous studies of C. difficile in food animals. Of cooked and uncooked meat products sold in Arizona, 42% were C. difficile positive, with toxigenic strains (ribotype 078, 73%; ribotype 027, 27%) (182). Following the emergence of ribotype 078 in humans, a pilot study was carried out in the Netherlands by the Food and Consumer Product Safety Authority to determine the occurrence of C. difficile in 500 consumable-meat samples from calves, pigs, sheep, turkeys, and chickens. The only positive samples were four samples from chicken (ribotypes 001, 003 [two samples], and 087). Similarly, of 82 meat samples collected from randomly selected retail shops in Sweden, C. difficile was isolated from only 2 (2.4%) (201). These results are clearly different from experiences reported for the United States and Canada. Notably, recent data from Canada show a very low prevalence of C. difficile (204), and a recent report suggested possible seasonal variation, with the highest prevalence in winter (166). It is clear that the epidemiology and significance of C. difficile in animals and food, and notably any implications for human CDI, require further investigation.
Antibiotic Risk Factor and Prescribing Intervention Studies
Prior exposure to antimicrobials is the main risk factor for CDI development. In general, expanded-spectrum and broad-spectrum cephalosporins and clindamycin are the most frequently implicated antibiotics in CDI, whereas the role of fluoroquinolones is less clear. However, the implication of almost every antibiotic in CDI development reflects widespread use and also the difficulty in ascribing the relative risk of CDI to specific antibiotics. The retrospective nature of many studies combined with noted risk factors such as polypharmacy, number of doses, and treatment duration (22, 29, 72, 133, 192, 194, 213, 215) may lead to interpretive problems. Crucially, exposure to C. difficile, which may be affected not only by where the patient is managed (including the relation of the place to that of other CDI cases) but also by the duration of hospital stay and infection control interventions, is also frequently overlooked when conclusions are drawn. The effects of exposure to C. difficile may well be exaggerated in the outbreak setting, which is typically when data on antimicrobial prescribing and CDI rates are collected. For all these reasons, odds ratios for the risk of CDI with individual antibiotics should be treated with caution (Table 1). Given the many potential confounding factors when determining CDI risk, it is not surprising that the use of antibiotics measured at the hospital level may not correlate with the likelihood of occurrence of CDI outbreaks (198, 208). When reviewing antibiotic risk data, it is important to distinguish between studies that identify apparent antibiotic risk factors for CDI and those where antimicrobial intervention strategies, typically comprising restrictive prescribing, have been employed to reduce the rate of infection.
TABLE 1.
Studies examining possible associations between fluoroquinolones and CDIa
| Study (reference) | Study yr | Setting | C. difficile ribotype involved | Quinolone(s) | Study design (no. of positive cases/total no. of cases) | Logistic regression analysis result(s) | Reported relative contribution to CDI cases (%) |
|---|---|---|---|---|---|---|---|
| Yip et al. (217) | 1998 | 300-bed U.S. tertiary-care hospital | Unknown | Ciprofloxacin | Retrospective case-control study (27/54) | Ciprofloxacin OR = 9.5 | |
| Cephalosporin OR = 6.7 | |||||||
| McCusker et al. (129) | 2001 | 778 beds, Veterans Affairs hospital, Baltimore, MD | Unknown | Levofloxacin, ciprofloxacin, gatifloxacin | Retrospective case-control study (30/60) | Fluoroquinolone OR = 12.7 | |
| Cephalosporin OR = 0.4 | |||||||
| Clindamycin OR = 2.2 | |||||||
| Gaynes et al. (71) | 2002 | 173-bed acute-care U.S. hospital | Unknown | Switch levofloxacin to gatifloxacin | Retrospective case-control study (37/59) | Clindamycin and increased duration of gatifloxacin therapy | |
| Muto et al. (139) | 2000-2001 | Pittsburgh, PA | Polyclonal | Levofloxacin | Retrospective case-control study (203/203) | Levofloxacin OR = 2.0 | 31 |
| Ceftriaxone OR = 5.4 | 6.7 | ||||||
| Clindamycin OR = 4.8 | 10 | ||||||
| Loo et al. (121) | 2004 | 12 hospitals in Quebec (8 university and 4 community) | 027 | Ciprofloxacin, gatifloxacin, moxifloxacin | Prospective matched case-control study (237/237) | Fluoroquinolone OR = 3.9 | |
| Cephalosporin OR = 3.8 | |||||||
| Pepin et al. (154) | 2003-2004 | Teaching hospital, Sherbrook, Canada | 027 | Ciprofloxacin, levofloxacin, gatifloxacin, moxifloxacin | Retrospective cohort (293/5,619) | Fluoroquinolone adjusted hazard ratio = 3.44 | 35.9 |
| Cephalosporin adjusted hazard ratio = 1.56-1.89 | 10 | ||||||
| Clindamycin adjusted hazard ratio = 1.77 | 1.5 | ||||||
| Kazakova et al. (98) | 2002-2003 | Community acute-care hospital in Maine | 027 | Levofloxacin, ciprofloxacin | Matched case-control study (68/127) | Fluoroquinolone OR = 3.22 | |
| Cephalosporin OR = 5.19 | |||||||
| McFarland et al. (134) | 2004 | 400-bed Veterans Affairs hospital, Seattle, WA | Unknown | Gatifloxacin | Retrospective matched case-control study (184/184) | Clindamycin OR = 29.9 | |
| Penicillin OR = 4.1 | |||||||
| Biller et al. (23) | 2003 | 320, acute-care, nonteaching U.S. hospital | 027 | Switch levofloxacin to moxifloxacin | Matched case-control study (50/100) | Moxifloxacin OR = 3.14 | |
| Cephalosporin OR = NS | |||||||
| Clindamycin OR = NS | |||||||
| Weiss et al. (207) | 2007 | Two 600-bed tertiary-care hospitals | 027 | Ciprofloxacin, levofloxacin, moxifloxacin | Case-control study (15/31) | Fluoroquinolone OR = 36.2 | |
| Cephalosporin OR = 19.1 | |||||||
| Debast et al. (50) | 2005 | 341-bed community hospital | 027 | Ciprofloxacin | Prospective case-control study (45/90), including extra control group with non-CDI diarrhea (109) | Fluoroquinolone OR = 28.8 | 33 |
| Cephalosporin OR = 7.8 | 56 | ||||||
| Combination OR = 57.5 |
NS, not significant.
C. difficile ribotype 027 strains appear to have acquired resistance to newer fluoroquinolones (moxifloxacin, levofloxacin, and gatifloxacin) by mutations in GyrA and GyrB, as historical (1980s to 1990s) C. difficile ribotype 027 isolates are susceptible to these antibiotics (130). Fluoroquinolone resistance can also be induced in vitro (183), and increased MICs of levofloxacin and/or moxifloxacin are associated with specific substitutions in GyrA and/or GyrB (183). It is plausible that the acquisition of fluoroquinolone resistance contributed to the increased spread of C. difficile ribotype 027 and possibly other C. difficile strains via selection pressure. Loo et al. (121) conducted a prospective case-control study in 12 Quebec, Canada, hospitals and found that CDI patients were more likely to have received fluoroquinolones (OR, 3.9; 95% CI, 2.3 to 6.6) or cephalosporins (OR, 3.8; 95% CI, 2.2 to 6.6) (121). Associations between CDI and fluoroquinolones, including ciprofloxacin, were described previously (31), but the emergence of ribotype 027 resulted in a number of new studies (summarized in Table 1). The odds ratio for fluoroquinolones calculated from the 11 studies shown in Table 1 was 12.8, whereas the odds ratio for cephalosporins was 5.1. C. difficile ribotype 027 was involved in six of the studies. No association with a specific fluoroquinolone was found, but most studies did not perform this analysis. A recent prospective case-control study reported a high risk of CDI due to ribotype 027 in patients who received a combination of a cephalosporin and a fluoroquinolone (OR, 57.5; 95% CI, 6.8 to 483.6) (50). The relative contribution of an antibiotic or class to causing CDI may be assessed by determining the proportion of cases that were attributable to the use of specific agents (i.e., the etiological fractions). However, as depicted in Table 1, only 3/11 studies provided such data, and therefore, the magnitude of the effect of specific antibiotics in selecting for C. difficile or in inducing CDI remains unclear. Pepin et al. found that long-duration fluoroquinolone therapy enhanced the risk of CDI (154). A recent case-control study also reported that ciprofloxacin usage for >7 days was a significant risk factor for CDI (adjusted OR, 3.72; 95% CI, 1.38 to 10.02; P = 0.019) (186).
The restricted use of certain high-risk antimicrobials is a commonly used strategy for reducing CDI rates. Antibiotic prescribing intervention studies have been used to examine prospectively the effects of a prescribing change upon CDI rates, although there are relatively few of these studies. A 2005 review of interventions to improve antibiotic prescribing practices found five studies designed to assess the impact of prescribing changes upon rates of CDI (48). Four studies showed that prescribing changes were associated with significant decreases in CDI rates, while the remaining study demonstrated a trend toward decreased CDI rates (34, 42, 100, 136, 148). Two studies examined the effect of restricting cephalosporin prescribing in planned interventions. Khan and Cheesbrough described a financially driven change in prescribing policy from cefotaxime to ceftriaxone, which was associated with an increase in CDI rates. A subsequent restriction of ceftriaxone use produced a modest decrease in CDI cases. However, a reduction in the proportion of C. difficile toxin-positive samples from 14.5 to 8.6% was observed when levofloxacin was substituted for ceftriaxone (100). Similarly, Carling et al. described the implementation of an antibiotic management program designed to minimize the inappropriate prescribing of broad-spectrum cephalosporins and reported sustained reduced CDI rates (34). The introduction of a low-risk antimicrobial to a formulary may not in itself produce a reduction in CDI rates. Wilcox et al. (212) described how the number of CDI cases did not decrease significantly after the introduction of piperacillin-tazobactam to a medicine formulary for the elderly until its use was accompanied by the restriction of cefotaxime. Numbers of CDI cases then decreased by 52% (P < 0.008). That study and one other study noted that increased cephalosporin prescribing in response to a shortage of piperacillin-tazobactam was associated with increases in CDI rates (3, 212). Those studies underline the necessity of removing or restricting the use of high-risk antibiotics as opposed to simply adding lower-risk agents to the formulary (212).
Similar methodological issues have affected studies performed since the emergence of C. difficile ribotype 027. Gaynes et al. described a CDI outbreak in a long-term-care facility caused by this strain that was apparently precipitated by a formulary switch from levofloxacin to gatifloxacin, with a decline in infection after reversion to levofloxacin treatment (71). In contrast, Biller et al. found that an outbreak apparently precipitated by a formulary change from levofloxacin to moxifloxacin was not controlled by reversion to levofloxacin (23). However, both studies described the implementation of infection control measures during the study period, which may have affected C. difficile exposure among patients and, therefore, CDI rates. McFarland et al. found that an increase in numbers of CDI cases thought to have been associated with a formulary change from levofloxacin to gatifloxacin was in line with seasonal variations in CDI rates (134). Interestingly, although fluoroquinolones had been identified retrospectively as a significant risk factor during a large outbreak of CDI in Quebec, Canada (155), CDI rates subsequently decreased in association with the antimicrobial restriction of narrow-spectrum (−21%), expanded-spectrum (−93%), and broad-spectrum (−79%) cephalosporins; clindamycin (−87%); macrolides (−78%); and ciprofloxacin (−29%); however, prescribing of respiratory fluoroquinolones (predominantly moxifloxacin) and piperacillin-tazobactam increased by +79% and +114%, respectively (195). It has been suggested that the combined implementation of improved infection control measures and judicious antimicrobial use, as opposed to the restriction of a specific antimicrobial, was responsible for eventually controlling this outbreak (20, 208). A recent interesting study examined the risk factors for CDI in cases due to both epidemic (C. difficile NAP1) and nonepidemic strains (95). CDI cases due to the epidemic strain (but not nonepidemic strains) were more likely than control patients to have received a fluoroquinolone upon univariate analysis. However, fluoroquinolone use was not significantly associated with CDI in a multivariable analysis. Also, while the outbreak was controlled after the complete restriction of fluoroquinolone prescribing, other factors, including a change in environmental-services contractors, may have played a key role. Thus, these contradictory data mean that it remains unclear what is the true contribution of fluoroquinolones (in the absence of other risk factors) to outbreaks of CDI caused by ribotype 027.
Prior antimicrobial therapy is a major risk factor for CDI because of the selection pressure for C. difficile strains and/or the induction of toxin production. Reports continue to emphasize the importance of multifaceted approaches (i.e., antimicrobial stewardship and enhanced infection control procedures) to the successful control of CDI outbreaks (50, 140, 209).
Alcohol-Based Hand Hygiene and Risk of CDI
C. difficile, like all sporulating bacteria, may initiate a conversion from metabolically active vegetative cells to dormant endospores when environmental conditions become adverse. Sporulation may occur potentially in response to nutrient limitation, exposure to antimicrobial disinfectants, or therapeutic antimicrobial agents (62, 67, 180, 211). The transmission of C. difficile in the nosocomial environment is believed to be due primarily to the ingestion of spores, which may have been acquired from other patients directly via the hands of health care workers or indirectly from inanimate objects. Thus, hand hygiene practices targeted at minimizing health care-associated infections (HCAI) are complicated by the intrinsic resistance of C. difficile spores to many disinfectants. Current World Health Organization (WHO) guidelines on hand hygiene (156) recommend a two-pronged approach to hand disinfection: (i) if exposure to sporulating pathogens is suspected, hand washing with soap and water is preferred, and (ii) in all other clinical situations, if hands are not visibly soiled, alcohol-based hand rubs (ABHR) are preferred for routine hand antisepsis (156). ABHR application is faster and easier than washing with soap and water and is easily accessible at the point of care; therefore, compliance with hand hygiene measures are potentially improved with this approach. Unfortunately, if patients are culture positive for C. difficile but asymptomatic, the implementation of the appropriate WHO hand hygiene strategy by health care workers is not straightforward. In this context, it has been shown, at least in the setting of a long-term-care facility affected by C. difficile ribotype 027, that skin and associated environmental contamination by the bacterium is commonly seen in asymptomatic carriers (160).
Given the inherent lack of activity of alcohol on spores, some have speculated that the enhanced use of ABHR may facilitate the transmission of C. difficile (15, 74). However, there are no robust data in the literature to support this hypothesis. Oughton and colleagues (145) performed an in vitro study using 10 volunteers whose hands were inoculated with a standard culture of a nontoxigenic C. difficile strain (1.4 × 105 CFU/ml, comprising 62% spore forms of the organism). Volunteers' hands were subsequently assigned randomly to one of five hand hygiene interventions, including an ABHR containing 70% (vol/vol) isopropanol, and C. difficile viable counts from hand washings were determined. The ABHR intervention was ineffective at reducing C. difficile counts compared with several of the other hand hygiene interventions (145). Reductions in viable counts of C. difficile spores have been demonstrated following ABHR application in laboratory studies (118). Leischner et al. evaluated the efficacies of chlorhexidine soap and water and three ABHR in removing C. difficile spores from artificially contaminated hands of volunteers. ABHR were less effective than chlorhexidine soap and water in reducing the C. difficile spore burden, but a decline in viable counts of 1.7 to 1.9 log10 CFU/ml was observed.
Despite the poor activity of ABHR against C. difficile spores in volunteer studies and their extensive implementation in the clinical setting, there are no convincing in vivo data to support the hypothesis of a resultant increased incidence of CDI. King (104) reported the results of a 3-month pilot study where ABHR were implemented in a 28-bed acute-care hospital ward. Interestingly, the C. difficile incidence increased following ABHR implementation (five new cases compared to zero cases in the preceding 12 months), but that study was limited to one small ward in a single institution and, therefore, was insufficiently powered to allow firm conclusions. Several more recent and larger in vivo studies evaluating CDI incidence following ABHR implementation have been performed (26, 94, 170, 200, 184, 199). Stone and colleagues reported the results of the first national hand hygiene campaign (“Clean Your Hands” campaign [CYHC]) involving 187 United Kingdom hospitals (185). The procurement of soap and ABHR and hospital bed occupancy data were monitored in relation to quarterly mandatory reports of methicillin-resistant Staphylococcus aureus (MRSA)/methicillin-sensitive S. aureus (MSSA) bacteremia and CDI. Interventions significantly associated with reduced CDI incidence were hand washing with soap and water and visitation of a Department of Health improvement team. The CDI incidence did not increase despite increased ABHR usage (185). In a recent study, Kaier et al. built a multivariate regression model to identify the dynamic relationship between antibiotic exposure, ABHR, and the incidence of MRSA and CDI (94). The increased usage of ABHR over the 44-month study period negatively impacted MRSA incidence but did not correlate (positively or negatively) with CDI incidence (94). ABHR are convenient for both health care workers and visitors and have been clearly proven to be efficacious in enhancing hand hygiene compliance and in reducing incidences of nosocomial infections. At an institutional level, the benefit obtained from increased hand hygiene compliance likely exceeds any risks related to the resistance of C. difficile spores to alcohol.
C. difficile Transmission Pressure
Increased length of hospital stay and number of hospital admissions continue to be identified as risk factors for CDI (7, 50, 70, 114, 134, 146). This could be explained simply by the increased risk of exposure to C. difficile (transmission pressure) via, for example, the hands of health care workers or indirectly from the hospital environment. Several studies have reported the presence of C. difficile in the hospital environment (61, 74, 160), and one study demonstrated increased environmental levels of C. difficile in a ward after it first opened to patient admissions despite there being no demonstrable C. difficile beforehand (61). In a recent whole-hospital-based study, the median C. difficile transmission pressure (termed “CDAD pressure” by those authors) was significantly higher for case patients with CDI than for controls (1.4 versus 0.3; P < 0.001) and notably remained an independent risk factor after adjustment for key potential confounders, including antimicrobial use. Of the 382 case patients examined, only one had a CDAD pressure of zero (56). However, “CDAD pressure” may be an uncertain measure because it lacks precision with respect to the exact time of potential exposure within the time frame of the length of stay. Thus, identical pressure indexes may be reached under conditions that represent quite distinct risks for acquiring CDI, for example, the timing of C. difficile exposure relative to other risk factors, such as admission/discharge day and antibiotic therapy (77).
Asymptomatic C. difficile carriage was not measured in the above-described studies, and thus, its contribution to C. difficile transmission pressure is unknown. It is arguable that a symptomatic CDI patient likely represents more of a cross-infection risk than an asymptomatic carrier. However, a recent study suggested that the asymptomatic C. difficile carriage rate for elderly patients in a long-term-care facility may be as high as 52% in outbreak settings, and crucially, such individuals outnumbered CDI cases 7-fold (160); these findings may have been exaggerated by the frequent occurrence of rectal incontinence. The skin of C. difficile carriers and their associated environmental surfaces were often contaminated by the bacterium, and spores were easily transferred to investigators' hands. Asymptomatic carriers could therefore represent a significant C. difficile transmission pressure, but corroboratory studies are needed here. It is nevertheless prudent to strive to optimize universal patient and environmental hygiene as a control measure to reduce C. difficile transmission pressure.
A detailed discussion of control measures to reduce C. difficile transmission pressure methods (effective barrier precautions during patient contact, use of isolation rooms, good hand hygiene practice, use of gloves and gowns, and appropriate environmental cleaning) is outside the limit of this review but can be found in United Kingdom Department of Health/Health Protection Agency (HPA) guidelines (51), Society for Healthcare Epidemiology of America/Infectious Diseases Society of America practice recommendations (57), and a European C. difficile Infection Control Group/ECDC report (202).
EVIDENCE FOR CHANGING CLINICAL VIRULENCE AND OUTCOME OF CDI
Increases in the frequency, severity, and recurrence rate of CDI were recognized in cases from Quebec, Canada, occurring since 2002 (121, 152, 153, 154). More complications were observed, such as toxic megacolon, severe disease requiring colectomy, shock, and death. These complications were associated with an age of >65 years, acquisition of infection in a hospital, peripheral leukocyte count of >20 × 109 leukocytes/liter, renal failure, and severe immunosuppression. The proportion of patients dying within 30 days after diagnosis increased from 4.7% in 1991 to 1992 to 13.8% in 2003 (P < 0.001). The cumulative mortality rate 12 months after diagnosis was significantly higher among patients with CDI (37%) than among controls (21%) (153). An impressive 1-year prospective surveillance study in 88 Quebec hospitals, performed in 2005, revealed that more severe disease was associated with ribotype 027 than with other types (87). Ribotype 027 was associated with both increased CDI incidence and more severe disease. CDI was the attributable cause of death in 2.6% of cases and contributed to death in another 4.5% of cases. The observation of more severe CDI associated with ribotype 027 was also made in Europe. In the Netherlands, 218 CDI cases caused by ribotype 027 were compared with 645 cases with CDI due to other ribotypes, mainly ribotypes 001 (18%) and 014 (7%) (76). Clear but nonsignificant trends were observed for more severe diarrhea (OR, 1.99; 95% CI, 0.83 to 4.73), higher attributable mortality (6.3% versus 1.2%; OR, 3.30; 95% CI, 0.41 to 26.4), and more recurrences (OR, 1.44; 95% CI, 0.94 to 2.20) in ribotype 027 cases. Lambert et al. compared the clinical characteristics of 211 patients with CDI due to ribotype 027 with 679 patients who had CDI due to other ribotypes in a large prospective study in Belgium (115); an age of over 80 years (OR, 1.6; P = 0.01) and the severity of the underlying condition, but not sex, were independent predictors of CDI-related death. Infection with ribotype 027 was associated with an OR of 1.9 (P = 0.041) for CDI-related death. In England, crude (death within 28 days) and early (death within 72 h) mortality rates were 23% and 11%, respectively, for CDI ribotype 027 cases, in comparison with 11% and 3%, respectively, for ribotype 106 cases (186). In Leeds, United Kingdom, 35 CDI cases caused by ribotype 027 were compared with 35 non-ribotype 027, age-, sex-, and location-matched cases (60). The duration or extent of diarrhea and frequency of abdominal pain, pyrexia, hypoalbuminemia, or leucocytosis did not differ between the two groups. However, there were more first-line metronidazole treatment failures in ribotype 027 cases (29% versus 3%; P = 0.03), and these were more likely to have an acute increase (>20 μM) in serum creatinine levels (20% versus 3%; P = 0.055). Crude mortality up to day 30 (25% in ribotype 027 cases versus 14% in controls), day 60 (29% versus 31%), and day 90 (34% versus 37%) appeared to reflect comorbidities. One small study was unable to demonstrate more severe CDI due to ribotype 027, but this was a retrospective analysis with no adjustment for comorbidities (137).
Given the experience of the changing clinical characteristics of CDI associated with ribotype 027, it is important to be alert for the potential emergence of new C. difficile strains. C. difficile ribotype 078 isolates carry known virulence markers similar to those present in ribotype 027 strains: the tcdA and tcdB binary toxin genes and a 39-bp deletion in tcdC in combination with a point mutation at position 184, resulting in a stop codon (8, 28). There is some evidence that the rate of CDI caused by ribotype 078 is increasing; a 2008 European surveillance study (16) found that 8% of cases were due to ribotype 078, compared with 3% in a 2005 study (14). In the Netherlands, the proportion of ribotype 078 increased from 3% in 2005 to 13% at the end of 2007, whereas the proportion of ribotype 027 decreased from 27% to 1%. An increase in the number of cases due to ribotype 078 has also been reported from France (169). Data from the National Reference Laboratory in the Netherlands indicate a clear trend for more severe diarrhea associated with ribotype 078 (77). Compared to CDI caused by ribotype 027, patients with CDI caused by ribotype 078 were significantly younger and had community-associated disease significantly more often. The presence of severe CDI (38.9% versus 40.0%) and the attributable mortality rate (3.8% versus 4.0%) were similar, whereas a complicated course was found significantly less frequently (9.6% versus 17.7%; OR, 0.20). In contrast to ribotype 027, outbreaks due to ribotype 078 were only sporadically found. Further data on potential emerging C. difficile ribotypes are needed. Clearly, this requires effective surveillance, including C. difficile culture, to be in place, which is underpinned by increased vigilance.
SUSCEPTIBILITY OF C. DIFFICILE TO METRONIDAZOLE AND VANCOMYCIN
In vitro antimicrobial resistance in C. difficile has been reported for several classes of antimicrobial agents that have been recognized as high risk for the induction of CDI (73). However, resistance to antimicrobial agents is not essential for CDI, since C. difficile may be susceptible to relatively high-risk antimicrobial agents, such as the aminopenicillins (66). Since the worldwide emergence of the apparently hypervirulent ribotype 027 C. difficile strain, many reports have documented increased severities of infection, higher rates of symptomatic recurrence (i.e., primary treatment failure), and significantly increased mortality rates (60, 109, 138, 152, 154). Metronidazole has long been the first-line therapy for CDI, and its reduced effectiveness was reported in a prospective observational study of 207 CDI patients, in which only 50% of patients were successfully treated and 22% continued to experience symptomatic CDI despite ≥10 days of treatment. Additionally, 28% of patients experienced symptomatic recurrence within 90 days (138). Similar observations of a reduced initial response to metronidazole and increased recurrent CDI were also reported in Quebec, Canada (155). Notably, however, while there have been occasional reports of resistance to metronidazole (CLSI breakpoint of 32 mg/liter) (12, 27, 149, 150, 178, 179, 216), metronidazole treatment failure has not been linked to antimicrobial resistance in C. difficile (172).
Several recent reports have highlighted that the epidemiology of metronidazole susceptibility in C. difficile may have changed. In a retrospective evaluation of 272 recent C. difficile isolates (2005 to 2006) submitted to the C. difficile Ribotyping Network for the United Kingdom (CDRN), we reported that 24.4% of C. difficile ribotype 001 isolates demonstrated metronidazole MICs of ≥6 mg/liter using a spiral gradient endpoint (SGE) screening method (P < 0.001) (9). These observations were not noted for strains of two other epidemic C. difficile ribotypes (ribotypes 027 and 106). It was clearly demonstrated that the experimental method affects metronidazole MICs in C. difficile, but reproducible MICs of ≥4 mg/liter (range, 4 to 16 mg/liter) were observed for all of the isolates highlighted by SGE, and these were statistically significantly elevated compared to a comparator group (n = 72) of historic (1995 to 2001) C. difficile isolates (9). Multilocus variable-number tandem-repeat analysis (MLVA) of C. difficile with reduced metronidazole susceptibility revealed two clonal complexes of related strains. In a larger, more recent study (2008 to 2009), we evaluated metronidazole and vancomycin susceptibilities of 1,010 C. difficile isolates submitted from multiple hospitals in England to the CDRN using both SGE and agar incorporation techniques (69). C. difficile PCR ribotypes 027 (NAP1), 106, and 001 comprised >50% of the isolates evaluated. Overall geometric mean metronidazole MICs of C. difficile ribotypes 027, 106, and 017 (1.73, 1.56, and 1.15 mg/liter, respectively) were statistically significantly higher than those of the 10 other most commonly encountered PCR ribotype groups (0.39 to 0.54 mg/liter; P < 0.0001). Similar observations of higher overall metronidazole MICs for epidemic C. difficile ribotypes were reported previously (28, 30). Metronidazole MICs for C. difficile ribotype 001 were statistically significantly lower than those demonstrated for a panel of isolates from 2005 to 2006 (P = 0.016); the proportion of isolates with MICs of ≥4 mg/liter was 54% versus 17%, respectively. Interestingly, significant increases in metronidazole MICs for C. difficile PCR ribotypes 027 and 106 were observed (P < 0.001), with 17% and 44% of isolates, respectively, demonstrating metronidazole MICs of ≥4 mg/liter. Additionally, 12 C. difficile isolates with vancomycin MICs of 4 mg/liter, comprising PCR ribotypes 106 (n = 6), 001 (n = 5), and 027 (n = 1), were observed (69), compared with only a single isolate in our prior study (9). In a similarly large susceptibility study of 1,080 stool samples from diagnostic laboratories in Ontario, Canada, Martin et al. reported 19 (1.8%) C. difficile isolates with metronidazole MICs of >8 mg/liter and 4 isolates with vancomycin MICs of 4 mg/liter (128). However, the C. difficile isolates (including PCR ribotypes 027 and 078) were only transiently resistant by Etest, with resistance apparently being lost upon repeated subculturing, which reflects data from previously reported in vitro studies (151). The potential clinical significance of reduced susceptibility to metronidazole in C. difficile isolates is unclear, but given the pharmacokinetic profile of metronidazole in humans, only a small elevation in the MIC could have a marked effect on the treatment efficacy in CDI, although there are no published reports to confirm this hypothesis. Mean metronidazole concentrations in feces of patients range between <0.25 and 9.5 mg/kg and are dependent on the inflammatory state of the colon (5, 24, 107). Therefore, C. difficile isolates with metronidazole MICs of ≥4 mg/liter may be unaffected by colonic concentrations of the drug. In contrast, C. difficile strains with vancomycin MICs of 4 to 8 mg/liter are unlikely to be clinically significant, as concentrations ranging between 520 and 2,200 mg/liter in feces of patients have been demonstrated (218).
The mechanism(s) of reduced susceptibility to metronidazole in C. difficile has not yet been identified, but Brazier et al. did not detect the presence of nim genes in the first metronidazole-resistant C. difficile strain (MIC = 16 mg/liter) isolated in the United Kingdom (27). Pelaez and colleagues reported that metronidazole resistance in Spanish C. difficile isolates was heterogeneous, inducible, and not related to the presence of nim genes (151), but whether these observations are reproducible with C. difficile isolates from other countries remains to be determined. As the predominant strains causing CDI continue to change, and given the potential for the clonal spread of strains with reduced susceptibility (9), there is continuing surveillance by reference laboratories worldwide to detect resistance emergence.
Biography
 J. Freeman, Ph.D., gained her postgraduate degree from the University of Leeds in 2001, working on Clostridium difficile[r]. She spent 4 years as a postdoctoral fellow there, working on C. difficile virulence and antimicrobial susceptibility and establishing a human gut model of C. difficile infection (CDI). She moved to Leeds Teaching Hospitals Trust in 2005 as a Clinical Scientist in Microbiology and works within the reference laboratory for the Clostridium Difficile Ribotyping Network for England and Northern Ireland (CDRN). She is involved in surveillance of antimicrobial susceptibility in C. difficile. She maintains an active research role into C. difficile pathogenesis and virulence, developing new models of CDI and evaluating new treatments for CDI.
J. Freeman, Ph.D., gained her postgraduate degree from the University of Leeds in 2001, working on Clostridium difficile[r]. She spent 4 years as a postdoctoral fellow there, working on C. difficile virulence and antimicrobial susceptibility and establishing a human gut model of C. difficile infection (CDI). She moved to Leeds Teaching Hospitals Trust in 2005 as a Clinical Scientist in Microbiology and works within the reference laboratory for the Clostridium Difficile Ribotyping Network for England and Northern Ireland (CDRN). She is involved in surveillance of antimicrobial susceptibility in C. difficile. She maintains an active research role into C. difficile pathogenesis and virulence, developing new models of CDI and evaluating new treatments for CDI.
 M. P. Bauer, MD, was trained in general internal medicine and then specialized in infectious diseases at Leiden University Medical Centre. Since 2007, he has been studying Clostridium difficile at the Centre for Infectious Diseases in the context of a Ph.D. program, focusing on epidemiology and the relationship between humoral immunity and recurrences while at the same time being involved in clinical work at the Department of Infectious Diseases.
M. P. Bauer, MD, was trained in general internal medicine and then specialized in infectious diseases at Leiden University Medical Centre. Since 2007, he has been studying Clostridium difficile at the Centre for Infectious Diseases in the context of a Ph.D. program, focusing on epidemiology and the relationship between humoral immunity and recurrences while at the same time being involved in clinical work at the Department of Infectious Diseases.
 S. D. Baines, Ph.D., received his graduate degree in medical microbiology from the University of Leeds in 2006. Subsequently, he was a postdoctoral research fellow in the same institution, working on the effects of antimicrobial agents on indigenous gut microfloras and Clostridium difficile using in vitro continuous-culture models. His main interests are gut microflora ecology, antimicrobial susceptibility testing, therapeutic interventions for C. difficile infection, and development of resistance to antimicrobial agents in C. difficile.
S. D. Baines, Ph.D., received his graduate degree in medical microbiology from the University of Leeds in 2006. Subsequently, he was a postdoctoral research fellow in the same institution, working on the effects of antimicrobial agents on indigenous gut microfloras and Clostridium difficile using in vitro continuous-culture models. His main interests are gut microflora ecology, antimicrobial susceptibility testing, therapeutic interventions for C. difficile infection, and development of resistance to antimicrobial agents in C. difficile.
 J. Corver, Ph.D., received his graduate degree in molecular virology from the Groningen University Medical School in 1998. Subsequently, he was a postdoctoral fellow at the California Institute of Technology, working on the structure of flaviviruses. After 3 years, he moved to the Leiden University Medical Center Department of Medical Microbiology. There he worked on the entry of enveloped viruses. Since 2007, he has been working on the molecular biology and biochemistry of Clostridium difficile. His main interests concern protein-protein interactions, gene regulation, and mechanisms of virulence.
J. Corver, Ph.D., received his graduate degree in molecular virology from the Groningen University Medical School in 1998. Subsequently, he was a postdoctoral fellow at the California Institute of Technology, working on the structure of flaviviruses. After 3 years, he moved to the Leiden University Medical Center Department of Medical Microbiology. There he worked on the entry of enveloped viruses. Since 2007, he has been working on the molecular biology and biochemistry of Clostridium difficile. His main interests concern protein-protein interactions, gene regulation, and mechanisms of virulence.
 W. N. Fawley received his Ph.D. in Microbiology in 2003 at Leeds University, United Kingdom. He has worked as a Clinical Scientist at the Leeds Teaching Hospitals Trust since 1992, where research and development work has centered around health care-associated infection, principally the epidemiology and typing of MRSA and Clostridium difficile. He is currently the lead Clinical Scientist at the reference laboratory for the Clostridium Difficile Ribotyping Network for England and Northern Ireland (CDRN).
W. N. Fawley received his Ph.D. in Microbiology in 2003 at Leeds University, United Kingdom. He has worked as a Clinical Scientist at the Leeds Teaching Hospitals Trust since 1992, where research and development work has centered around health care-associated infection, principally the epidemiology and typing of MRSA and Clostridium difficile. He is currently the lead Clinical Scientist at the reference laboratory for the Clostridium Difficile Ribotyping Network for England and Northern Ireland (CDRN).
 B. Goorhuis, MD, was trained in general internal medicine and is currently specializing in infectious diseases at the Leiden University Medical Centre. Since 2006, he has been studying Clostridium difficile at the department of Medical Microbiology and the Centre for Infectious Diseases, in the context of a Ph.D. program. His research focuses on epidemiology, aimed at subtype-specific risk factors of Clostridium difficile and infections in outbreak situations versus endemic settings.
B. Goorhuis, MD, was trained in general internal medicine and is currently specializing in infectious diseases at the Leiden University Medical Centre. Since 2006, he has been studying Clostridium difficile at the department of Medical Microbiology and the Centre for Infectious Diseases, in the context of a Ph.D. program. His research focuses on epidemiology, aimed at subtype-specific risk factors of Clostridium difficile and infections in outbreak situations versus endemic settings.
 E. J. Kuijper, MD, Ph.D., medical microbiologist, is the head of the Department of Experimental Microbiology at the Leiden University Medical Center. He introduced molecular biology and mass spectrometry in diagnostics of bacterial diseases and started a research project on Clostridium difficile with interest in epidemiology and pathogenesis. He is also the head of the National Reference Laboratory of Clostridium difficile, a collaboration of the LUMC with the Center for Infectious Control, National Institute of Health and the Environment (Bilthoven, Netherlands).
E. J. Kuijper, MD, Ph.D., medical microbiologist, is the head of the Department of Experimental Microbiology at the Leiden University Medical Center. He introduced molecular biology and mass spectrometry in diagnostics of bacterial diseases and started a research project on Clostridium difficile with interest in epidemiology and pathogenesis. He is also the head of the National Reference Laboratory of Clostridium difficile, a collaboration of the LUMC with the Center for Infectious Control, National Institute of Health and the Environment (Bilthoven, Netherlands).
 M. H. Wilcox, MD, is a Consultant Microbiologist, Head of Microbiology, and Clinical Director of Pathology at the Leeds Teaching Hospitals; Professor of Medical Microbiology at the University of Leeds; and the Lead on Clostridium difficile for the Health Protection Agency (HPA) in England. He is a member of the Department of Health's Antimicrobial Resistance and Healthcare Associated Infection (ARHAI) Committee and the HPA's Programme Board on Healthcare-Associated Infection (HCAI) and Antimicrobial Resistance. He is an advisor to the Department of Health in England on HCAIs, the Technology Strategy Board on HCAI diagnostics, the EPIC project, the Health Technology Assessment program on HCAI, and the Wellcome Trust. He is a member of United Kingdom, European, and U.S. working groups on C. difficile infection (CDI). Professor Wilcox's research team's projects include several areas of health care-associated infection, in particular CDI, staphylococcal infection, and the clinical development of new antimicrobial agents.
M. H. Wilcox, MD, is a Consultant Microbiologist, Head of Microbiology, and Clinical Director of Pathology at the Leeds Teaching Hospitals; Professor of Medical Microbiology at the University of Leeds; and the Lead on Clostridium difficile for the Health Protection Agency (HPA) in England. He is a member of the Department of Health's Antimicrobial Resistance and Healthcare Associated Infection (ARHAI) Committee and the HPA's Programme Board on Healthcare-Associated Infection (HCAI) and Antimicrobial Resistance. He is an advisor to the Department of Health in England on HCAIs, the Technology Strategy Board on HCAI diagnostics, the EPIC project, the Health Technology Assessment program on HCAI, and the Wellcome Trust. He is a member of United Kingdom, European, and U.S. working groups on C. difficile infection (CDI). Professor Wilcox's research team's projects include several areas of health care-associated infection, in particular CDI, staphylococcal infection, and the clinical development of new antimicrobial agents.
REFERENCES
- 1.Al-Nassir, W. N., A. K. Sethi, M. M. Riggs, G. S. Bobulsky, R. L. P. Jump, and C. J. Donskey. 2008. A comparison of clinical and microbiologic response to treatment of Clostridium difficile-associated disease with metronidazole and vancomycin. Clin. Infect. Dis. 47:56-62. [DOI] [PubMed] [Google Scholar]
- 2.al Saif, N., and J. S. Brazier. 1996. The distribution of Clostridium difficile in the environment of South Wales. J. Med. Microbiol. 45:133-137. [DOI] [PubMed] [Google Scholar]
- 3.Alston, W. K., and J. W. Ahern. 2004. Increase in the rate of nosocomial Clostridium difficile-associated diarrhoea during shortages of piperacillin-tazobactam and piperacillin. J. Antimicrob. Chemother. 53:549-550. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Perez, S., J. L. Blanco, E. Bouza, P. Alba, X. Gibert, J. Maldonado, and M. E. Garcia. 2009. Prevalence of Clostridium difficile in diarrhoeic and non-diarrhoeic piglets. Vet. Microbiol. 137:302-305. [DOI] [PubMed] [Google Scholar]
- 5.Arabi, Y., F. Dimock, D. W. Burdon, J. Alexander-Williams, and M. R. Keighley. 1979. Influence of neomycin and metronidazole on colonic microflora of volunteers. J. Antimicrob. Chemother. 5:531-537. [DOI] [PubMed] [Google Scholar]
- 6.Arroyo, L. G., S. A. Kruth, B. M. Willey, H. R. Staempfli, D. E. Low, and J. S. Weese. 2005. PCR ribotyping of Clostridium difficile isolates originating from human and animal sources. J. Med. Microbiol. 54:163-166. [DOI] [PubMed] [Google Scholar]
- 7.Asha, N. J., D. Tompkins, and M. H. Wilcox. 2006. Comparative analysis of prevalence, risk factors, and molecular epidemiology of antibiotic-associated diarrhea due to Clostridium difficile, Clostridium perfringens, and Staphylococcus aureus. J. Clin. Microbiol. 44:2785-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacci, S., G. St-Martin, B. Olesen., B. Bruun, K. E. Olsen, E. M. Nielsen, and K. Mølbak. 2009. Outbreak of Clostridium difficile 027 in North Zealand, Denmark, 2008-2009. Euro Surveill. 14(16):pii=19183. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19183. [PubMed] [Google Scholar]
- 9.Baines, S. D., R. O'Connor, J. Freeman, W. N. Fawley, C. Harmanus, P. Mastrantonio, E. J. Kuijper, and M. H. Wilcox. 2008. Emergence of reduced susceptibility to metronidazole in Clostridium difficile. J. Antimicrob. Chemother. 62:1046-1052. [DOI] [PubMed] [Google Scholar]
- 10.Bakri, M. M., D. J. Brown, J. P. Butcher, and A. D. Sutherland. 2009. Clostridium difficile in ready-to-eat salads, Scotland. Emerg. Infect. Dis. 15:817-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balassiano, I. T., K. R. Miranda, R. F. Boente, H. Pauer, I. C. Oliveira, J. Santos-Filho, E. L. Amorim, G. A. Caniné, C. F. Souza, M. Z. Gomes, E. O. Ferreira, J. S. Brazier, and R. M. Domingues. 2009. Characterization of Clostridium difficile strains isolated from immunosuppressed inpatients in a hospital in Rio de Janeiro, Brazil. Anaerobe 15:61-64. [DOI] [PubMed] [Google Scholar]
- 12.Barbut, F., D. Decre, B. Burghoffer, D. Lesage, F. Delisle, V. Lalande, M. Delmee, V. Avesani, N. Sano, C. Coudert, and J. C. Petit. 1999. Antimicrobial susceptibilities and serogroups of clinical strains of Clostridium difficile isolated in France in 1991 and 1997. Antimicrob. Agents Chemother. 43:2607-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbut, F., M. Delmee, J. S. Brazier, J. C. Petit, I. R. Poxton, M. Rupnik, V. Lalande, C. Schneider, P. Mastrantonio, R. Alonso, E. Kuipjer, and M. Tvede. 2003. A European survey of diagnostic methods and testing protocols for Clostridium difficile. Clin. Microbiol. Infect. 9:989-996. [DOI] [PubMed] [Google Scholar]
- 14.Barbut, F., P. Mastrantonio, M. Delmée, J. Brazier, E. Kuijper, and I. Poxton. 2007. Prospective study of Clostridium difficile-associated disease in Europe with phenotypic and genotypic characterization of the isolates. Clin. Microbiol. Infect. 13:1048-1057. [DOI] [PubMed] [Google Scholar]
- 15.Bartlett, J. G. 2006. The new epidemic of Clostridium difficile-associated enteric disease. Ann. Intern. Med. 145:758-764. [DOI] [PubMed] [Google Scholar]
- 16.Bauer, M. P., D. W. Notermans, B. H. B. van Benthem, M. H. Wilcox, D. L. Monnet, J. T. van Dissel, et al. 2009. First results of the European Clostridium difficile infection survey (ECDIS), abstr. O-85. Abstr. 19th Eur. Congr. Clin. Microbiol. Infect. Dis.
- 17.Bauer, M. P., D. Veenendaal, L. Verhoef, P. Bloembergen, J. T. van Dissel, and E. J. Kuijper. 2009. Clinical and microbiological characteristics of community-onset Clostridium difficile infection in the Netherlands. Clin. Microbiol. Infect. 15:1087-1092. [DOI] [PubMed] [Google Scholar]
- 18.Båverud, V. 2002. Clostridium difficile infections in animals with special reference to the horse. A review. Vet. Q. 24:203-219. [DOI] [PubMed] [Google Scholar]
- 19.Beaugerie, L., A. Flahault, F. Barbut, P. Atlan, V. Lalande, P. Cousin, M. Cadilhac, and J. C. Petit. 2003. Antibiotic-associated diarrhoea and Clostridium difficile in the community. Aliment. Pharmacol. Ther. 17:905-912. [DOI] [PubMed] [Google Scholar]
- 20.Beaulieu, M., D. J. G. Thirion, D. Williamson, and G. Pichette. 2006. Clostridium difficile-associated diarrhoea outbreaks: the name of the game is isolation and cleaning. Clin. Infect. Dis. 42:725. [DOI] [PubMed] [Google Scholar]
- 21.Benson, L., X. Song, J. Campos, and N. Singh. 2007. Changing epidemiology of Clostridium difficile-associated disease in children. Infect. Control Hosp. Epidemiol. 28:1233-1235. [DOI] [PubMed] [Google Scholar]
- 22.Bignardi, G. E. 1998. Risk factors for Clostridium difficile infection. J. Hosp. Infect. 40:1-15. [DOI] [PubMed] [Google Scholar]
- 23.Biller, P., B. Shank, L. Lind, M. Brennan, L. Tkatch, G. Killgore, A. Thompson, and L. C. McDonald. 2007. Moxifloxacin therapy as a risk factor for Clostridium difficile-associated disease during an outbreak: attempts to control a new epidemic strain. Infect. Control Hosp. Epidemiol. 28:198-201. [DOI] [PubMed] [Google Scholar]
- 24.Bolton, R. P., and M. A. Culshaw. 1986. Faecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficile. Gut 27:1169-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouza, E., A. Martin, R. J. Van den Berg, and E. J. Kuijper. 2008. Laboratory-acquired Clostridium difficile polymerase chain reaction ribotype 027: a new risk for laboratory workers? Clin. Infect. Dis. 47:1493-1494. [DOI] [PubMed] [Google Scholar]
- 26.Boyce, J. M., C. Ligi, C. Kohan, D. Dumigan, and N. L. Havill. 2006. Lack of association between the increased incidence of Clostridium difficile-associated disease and the increasing use of alcohol-based hand rubs. Infect. Control Hosp. Epidemiol. 27:479-483. [DOI] [PubMed] [Google Scholar]
- 27.Brazier, J. S., W. Fawley, J. Freeman, and M. H. Wilcox. 2001. Reduced susceptibility of Clostridium difficile to metronidazole. J. Antimicrob. Chemother. 48:741-742. [DOI] [PubMed] [Google Scholar]
- 28.Brazier, J. S. 2008. Antibiotic susceptibility patterns in Europe, abstr. S-233. Abstr. 18th Eur. Congr. Clin. Microbiol. Infect. Dis., Barcelona, Spain.
- 29.Brown, E., G. H. Talbot, P. Axelrod, M. Provencher, and C. Hoegg. 1990. Risk factors for Clostridium difficile toxin-associated diarrhea. Infect. Control Hosp. Epidemiol. 11:283-290. [DOI] [PubMed] [Google Scholar]
- 30.Burns, P., M. Wooton, V. Hall, J. S. Brazier, and R. Howe. 2007. Antimicrobial susceptibility of epidemic and nonepidemic strains of Clostrialdium difficile, abstr. C2-2046. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL.
- 31.Cain, D. B., and M. E. O'Connor. 1990. Pseudomembranous colitis associated with ciprofloxacin. Lancet 336:946. [DOI] [PubMed] [Google Scholar]
- 32.Camacho-Ortiz, A., A. Ponce-de-León, and J. Sifuentes-Osornio. 2009. Clostridum difficile associated disease in Latin America. Gac. Med. Mex. 145:223-229. (In Spanish.) [PubMed] [Google Scholar]
- 33.Campbell, R. J., L. Giljahn, K. Machesky, K. Cibulskas-White, L. M. Lane, K. Porter, J. O. Paulson, F. W. Smith, and L. C. McDonald. 2009. Clostridium difficile infection in Ohio hospitals and nursing homes during 2006. Infect. Control Hosp. Epidemiol. 30:526-533. [DOI] [PubMed] [Google Scholar]
- 34.Carling, P., T. Fung, A. Killion, N. Terrin, and M. Barza. 2003. Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years. Infect. Control Hosp. Epidemiol. 24:699-706. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. 2005. Severe Clostridium difficile associated disease in populations previously at low risk—four states, 2005. MMWR Morb. Mortal. Wkly. Rep. 54:1201-1205. [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. 2008. Surveillance for community-associated Clostridium difficile—Connecticut, 2006. MMWR Morb. Mortal. Wkly. Rep. 57:340-343. [PubMed] [Google Scholar]
- 37.Chandler, R. E., K. Hedberg, and P. R. Cieslak. 2007. Clostridium difficile-associated disease in Oregon: increasing incidence and hospital-level risk factors. Infect. Control Hosp. Epidemiol. 28:116-122. [DOI] [PubMed] [Google Scholar]
- 38.Cheknis, A. K., W. E. Zukowski, L. A. Petrella, K. J. Nagaro, S. P. Sambol, I. Figueroa, L. Q. Li, E. Perdue, S. P. Gelone, S. Johnson, and D. N. Gerding. 2009. Prevalence of REA types of Clostridium difficile from U.S. hospitals (2006-2009), abstr. K-2075. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA.
- 39.Cheng, V. C., W. C. Yam, J. F. Chan, K. K. To, P. L. Ho, and K. Y. Yuen. 2009. Clostridium difficile ribotype 027 arrives in Hong Kong. Int. J. Antimicrob. Agents 34:492-493. [DOI] [PubMed] [Google Scholar]
- 40.Cheng, Y., J. Lu, S. Yan, H. Jia, and W. Li. 2009. Study on toxin characteristics of Clostridium difficile isolated from hospital. Dis. Surveill. 24:193-196. (In Chinese.) [Google Scholar]
- 41.Chopra, T., L. Bernabela, L. Johnson, S. McNamara, S. Donabedian, M. Perri, H. Salimnia, M. Amjad, C. Pranatharthi, K. Kaye, and G. Alangaden. 2009. Molecular and epidemiological characterization of Clostridium difficile strains in a tertiary-care hospital, abstr. K-2080. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA.
- 42.Climo, M. W., D. S. Israel, E. S. Wong, D. Williams, P. Coudron, and S. M. Markowitz. 1998. Hospital-wide restriction of clindamycin: effect on the incidence of Clostridium difficile-associated diarrhea and cost. Ann. Intern. Med. 128:989-995. [DOI] [PubMed] [Google Scholar]
- 43.Cloten, J., S. Kruth, L. Arroyo, and J. S. Weese. 2008. Prevalence and risk factors for Clostridium difficile colonization in dogs and cats hospitalized in an intensive care unit. Vet. Microbiol. 129:209-214. [DOI] [PubMed] [Google Scholar]
- 44.Commission for Healthcare Audit and Inspection. 2006. Investigation into outbreaks of Clostridium difficile at Stoke Mandeville Hospital, Buckinghamshire Hospitals NHS Trust. Commission for Healthcare Audit and Inspection, London, United Kingdom. http://www.cqc.org.uk/_db/_documents/Stoke_Mandeville.pdf.
- 45.Crobach, M. J., O. M. Dekkers, M. H. Wilcox, and E. J. Kuijper. 2009. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): data review and recommendations for diagnosing Clostridium difficile-infection (CDI). Clin. Microbiol. Infect. 15:1053-1066. [DOI] [PubMed] [Google Scholar]
- 46.Dallal, R. M., B. G. Harbrecht, A. J. Boujoukas, C. A. Sirio, L. M. Farkas, K. K. Lee, and R. L. Simmons. 2002. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann. Surg. 235:363-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dalton, B. R., T. Lye-Maccannell, E. A. Henderson, D. R. Maccannell, and T. J. Louie. 2009. Proton pump inhibitors (PPI) significantly increase the risk of Clostridium difficile infection in a low endemicity, non-outbreak hospital setting: optimizing risk and benefit of PPI therapy. Aliment. Pharmacol. Ther. 29:626-634. [DOI] [PubMed] [Google Scholar]
- 48.Davey, P., E. Brown, L. Fenelon, R. Finch, I. Gould, G. Hartman, A. Holmes, C. Ramsay, E. Taylor, M. Wilcox, and P. Wiffen. 2005. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 19:CD003543. [DOI] [PubMed] [Google Scholar]
- 49.Debast, S. B., L. A. van Leengoed, A. Goorhuis, C. Harmanus, E. J. Kuijper, and A. A. Bergwerff. 2009. Clostridium difficile PCR ribotype 078 toxinotype V found in diarrhoeal pigs identical to isolates from affected humans. Environ. Microbiol. 11:505-511. [DOI] [PubMed] [Google Scholar]
- 50.Debast, S. B., N. Vaessen, A. Choudry, E. A. J. Wiegers-Ligtvoet, R. J. van den Berg, and E. J. Kuijper. 2009. Successful combat of an outbreak due to Clostridium difficile PCR ribotype 027 and recognition of specific risk factors. Clin. Microbiol. Infect. 15:427-434. [DOI] [PubMed] [Google Scholar]
- 51.Department of Health and Health Protection Agency. 2009. Clostridium difficile infection: how to deal with the problem. Department of Health and Health Protection Agency, London, United Kingdom. http://www.hpa.nhs.uk/web/HPAwebFile/HPAweb_C/1232006607827.
- 52.Dial, S., J. A. C. Delaney, A. N. Barkun, and S. Suissa. 2005. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA 294:2989-2995. [DOI] [PubMed] [Google Scholar]
- 53.Dial, S., A. Kezouh, A. Dascal, A. Barkun, and S. Suissa. 2008. Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile infection. CMAJ 179:767-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drudy, D., B. Goorhuis, D. Bakker, L. Kyne, R. van den Berg, L. Fenelon, S. Fanning, and E. J. Kuijper. 2008. Clindamycin-resistant clone of Clostridium difficile PCR ribotype 027, Europe. Emerg. Infect. Dis. 14:1485-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dubberke, E. R., K. A. Reske, Y. Yan, M. A. Olsen, L. C. McDonald, and V. J. Fraser. 2007. Clostridium difficile-associated disease in a setting of endemicity: identification of novel risk factors. Clin. Infect. Dis. 45:1543-1549. [DOI] [PubMed] [Google Scholar]
- 56.Dubberke, E. R., K. A. Reske, M. A. Olsen, K. M. McMullen, J. L. Mayfield, L. C. McDonald, and V. J. Fraser. 2007. Evaluation of Clostridium difficile-associated disease pressure as a risk factor for C. difficile-associated disease. Arch. Intern. Med. 167:1092-1097. [DOI] [PubMed] [Google Scholar]
- 57.Dubberke, E. R., D. N. Gerding, D. Classen, K. M. Arias, K. Podgorny, D. J. Anderson, H. Burstin, D. P. Calfee, S. E. Coffin, V. Fraser, F. A. Griffin, P. Gross, K. S. Kaye, M. Klompas, E. Lo, J. Marschall, L. A. Mermel, L. Nicolle, D. A. Pegues, T. M. Perl, S. Saint, C. D. Salgado, R. A. Weinstein, R. Wise, and D. S. Yokoe. 2008. SHEA/IDSA practise recommendations: strategies to prevent Clostridium difficile infections in acute care hospitals. Infect. Control Hosp. Epidemiol. 29(Suppl. 1):S81-S92. [DOI] [PubMed] [Google Scholar]
- 58.Dubberke, E. R., A. M. Butler, B. Hota, Y. M. Khan, J. E. Mangino, J. Mayer, K. J. Popovich, K. B. Stevenson, D. S. Yokoe, L. C. McDonald, J. Jernigan, V. J. Fraser, and the Prevention Epicenters Program from the Centers for Disease Control and Prevention. 2009. Multicenter study of the impact of community-onset Clostridium difficile infection on surveillance for C. difficile infection. Infect. Control Hosp. Epidemiol. 30:518-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eastwood, K., P. Else, A. Charlett, and M. Wilcox. 2009. Comparison of nine commercially available Clostridium difficile toxin detection assays, a real-time PCR assay for C. difficile tcdB, and a glutamate dehydrogenase detection assay to cytotoxin testing and cytotoxigenic culture methods. J. Clin. Microbiol. 47:3211-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ellames, D., M. Wilcox, W. Fawley, J. Freeman, and C. Smith. 2007. Comparison of risk factors and outcome of cases of Clostridium difficile infection due to ribotype 027 versus other types, abstr. K-605. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL.
- 61.Fawley, W. N., and M. H. Wilcox. 2001. Molecular epidemiology of Clostridium difficile infection. Epidemiol. Infect. 126:343-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fawley, W. N., S. Underwood, J. Freeman, S. D. Baines, K. Saxton, K. Stephenson, R. C. Owens, Jr., and M. H. Wilcox. 2007. Efficacy of hospital cleaning agents and germicides against epidemic Clostridium difficile strains. Infect. Control Hosp. Epidemiol. 28:920-925. [DOI] [PubMed] [Google Scholar]
- 63.Fawley, W. N., J. Freeman, C. Smith, C. Harmanus, R. J. van den Berg, E. J. Kuijper, and M. H. Wilcox. 2008. Use of highly discriminatory fingerprinting to analyze clusters of Clostridium difficile infection cases due to epidemic ribotype 027 strains. J. Clin. Microbiol. 46:954-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fenner, L., A. F. Widmer, A. Stranden, M. Conzelmann, A. Goorhuis, C. Harmanus, E. J. Kuijper, and R. Frei. 2008. First cluster of clindamycin-resistant Clostridium difficile PCR-ribotype 027 associated disease in Switzerland. Clin. Microbiol. Infect. 14:514-515. [DOI] [PubMed] [Google Scholar]
- 65.Fraser, T. G., C. Fatica, and S. M. Gordon. 2009. Necessary but not sufficient: a comparison of surveillance definitions of Clostridium difficile-associated diarrhea. Infect. Control Hosp. Epidemiol. 30:377-379. [DOI] [PubMed] [Google Scholar]
- 66.Freeman, J., and M. H. Wilcox. 1999. Antibiotics and Clostridium difficile. Microbes Infect. 1:377-384. [DOI] [PubMed] [Google Scholar]
- 67.Freeman, J., and M. H. Wilcox. 2000. Does antibiotic exposure affect sporulation of an epidemic Clostridium difficile strain?, abstr. 950. Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., Toronto, Ontario, Canada.
- 68.Freeman, J., W. N. Fawley, S. Baines, and M. Wilcox. 2006. Measurement of toxin production by Clostridium difficile. Lancet 367:982-983. [DOI] [PubMed] [Google Scholar]
- 69.Freeman, J., W. N. Fawley, J. Hobbs, P. Else, P. Verity, P. Parnell, S. D. Baines, and M. H. Wilcox. 2009. Decreasing metronidazole susceptibility in epidemic Clostridium difficile strains, abstr. C2-1964. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA.
- 70.Garey, K. W., T. K. Dao-Tran, Z. D. Jiang, M. P. Price, L. O. Gentry, and H. L. Dupont. 2008. A clinical risk index for Clostridium difficile infection in hospitalised patients receiving broad-spectrum antibiotics. J. Hosp. Infect. 70:142-147. [DOI] [PubMed] [Google Scholar]
- 71.Gaynes, R., D. Rimland, E. Killum, H. K. Lowery, T. M. Johnson II, G. Killgore, and F. C. Tenover. 2004. Outbreak of Clostridium difficile infection in a long-term care facility: association with gatifloxacin use. Clin. Infect. Dis. 38:640-645. [DOI] [PubMed] [Google Scholar]
- 72.Gerding, D. N., M. M. Olson, L. R. Peterson, and J. T. Lee. 1986. Clostridium difficile-associated diarrhea and colitis in adults. A prospective case-controlled epidemiologic study. Arch. Intern. Med. 146:95-100. [PubMed] [Google Scholar]
- 73.Gerding, D. N. 2004. Clindamycin, cephalosporins, fluoroquinolones, and Clostridium difficile-associated diarrhea: this is an antimicrobial resistance problem. Clin. Infect. Dis. 38:646-648. [DOI] [PubMed] [Google Scholar]
- 74.Gerding, D. N., C. A. Muto, and R. C. Owens, Jr. 2008. Measures to control and prevent Clostridium difficile infection. Clin. Infect. Dis. 46(Suppl. 1):S43-S49. [DOI] [PubMed] [Google Scholar]
- 75.Geric, B., R. J. Carman, M. Rupnik, C. W. Genheimer, S. P. Sambol, D. M. Lyerly, D. N. Gerding, and S. Johnson. 2006. Binary toxin-producing, large clostridial toxin-negative Clostridium difficile strains are enterotoxic but do not cause disease in hamsters. J. Infect. Dis. 193:1143-1150. [DOI] [PubMed] [Google Scholar]
- 76.Goorhuis, A., T. Van der Kooi, N. Vaessen, F. W. Dekker, R. Van den Berg, C. Harmanus, S. Van den Hof, D. W. Notermans, and E. J. Kuijper. 2007. Spread and epidemiology of Clostridium difficile polymerase chain reaction ribotype 027/toxinotype III in the Netherlands. Clin. Infect. Dis. 45:695-703. [DOI] [PubMed] [Google Scholar]
- 77.Goorhuis, A., D. Bakker, J. Corver, S. B. Debast, C. Harmanus, D. W. Notermans, A. A. Bergwerff, F. W. Dekker, and E. J. Kuijper. 2008. Emergence of Clostridium difficile infection due to a new hypervirulent strain, polymerase chain reaction ribotype 078. Clin. Infect. Dis. 47:162-170. [DOI] [PubMed] [Google Scholar]
- 78.Goorhuis, A., M. C. Legaria, R. J. van den Berg, C. Harmanus, C. H. Klaassen, J. S. Brazier, G. Lumelsky, and E. J. Kuijper. 2009. Application of multiple-locus variable-number tandem-repeat analysis to determine clonal spread of toxin A-negative Clostridium difficile in a general hospital in Buenos Aires, Argentina. Clin. Microbiol. Infect. 15:1080-1086. [DOI] [PubMed] [Google Scholar]
- 79.Gravel, D., M. Miller, A. Simor, G. Taylor, M. Gardam, A. McGeer, J. Hutchinson, D. Moore, S. Kelly, D. Boyd, and M. Mulvey. 2009. Health care-associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: a Canadian Nosocomial Infection Surveillance Program study. Clin. Infect. Dis. 48:568-576. [DOI] [PubMed] [Google Scholar]
- 80.Health Protection Agency. 2009. Clostridium difficile Ribotyping Network for England and Northern Ireland: 2008/09 report. Health Protection Agency, London, United Kingdom. http://www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1258560554236.
- 81.Health Protection Agency. 2009. Results of the mandatory Clostridium difficile reporting scheme. Health Protection Agency, London, United Kingdom. http://www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1195733750761.
- 82.Health Protection Agency. 2009. Voluntary surveillance of Clostridium difficile in England, Wales and Northern Ireland, 2008. Health Protection Agency, London, United Kingdom. http://www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1254510474808.
- 83.Heap, J. T., O. J. Pennington, S. T. Cartman, G. P. Carter, and N. P. Minton. 2007. The ClosTron: a universal gene knock-out system for the genus Clostridium. J. Microbiol. Methods 70:452-464. [DOI] [PubMed] [Google Scholar]
- 84.Henrich, T. J., D. Krakower, A. Bitton, and D. S. Yokoe. 2009. Clinical risk-factors for severe Clostridium difficile-associated disease. Emerg. Infect. Dis. 15:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hensgens, M. P., A. Goorhuis, D. W. Notermans, B. H. van Benthem, and E. J. Kuijper. 2009. Decrease of hypervirulent Clostridium difficile PCR ribotype 027 in the Netherlands. Euro Surveill. 14(45):pii=19402. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19402. [DOI] [PubMed]
- 86.Huang, H., H. Fang, A. Weintraub, and C. E. Nord. 2009. Distinct ribotypes and rates of antimicrobial drug resistance in Clostridium difficile from Shanghai and Stockholm. Clin. Microbiol. Infect. 15:1170-1173. [DOI] [PubMed] [Google Scholar]
- 87.Hubert, B., V. G. Loo, A. M. Bourgault, L. Poirier, A. Dascal, E. Fortin, M. Dionne, and M. Lorange. 2007. A portrait of the geographic dissemination of the Clostridium difficile North American pulsed-field type 1 strain and the epidemiology of C. difficile-associated disease in Quebec. Clin. Infect. Dis. 44:238-244. [DOI] [PubMed] [Google Scholar]
- 88.Indra, A., S. Huhulescu, P. Hasenberger, D. Schmid, C. Alfery. R. Wuerzner, M. Fille, K. Gattringer, E. Kuijper, and F. Allerberger. 2006. First isolation of Clostridium difficile PCR ribotype 027 in Austria. Euro Surveill. 11(9):E060914.3. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=3046. [DOI] [PubMed] [Google Scholar]
- 89.Indra, A., S. Huhulescu, A. Fiedler, S. Kernbichler, M. Blaschitz, and F. Allerberger. 2009. Outbreak of Clostridium difficile 027 infection in Vienna, Austria 2008-2009. Euro Surveill. 14(17):pii=19186. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19186. [PubMed]
- 90.Ingebretsen, A., G. Hansen, C. Harmanus, and E. J. Kuijper. 2008. First confirmed cases of Clostridium difficile PCR ribotype 027 in Norway. Euro Surveill. 13(2):pii=8011. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=8011. [PubMed]
- 91.Jhung, M. A., A. D. Thompson, G. E. Killgore, W. E. Zukowski, G. Songer, M. Warny, S. Johnson, D. N. Gerding, L. C. McDonald, and B. M. Limbago. 2008. Toxinotype V Clostridium difficile in humans and food animals. Emerg. Infect. Dis. 14:1039-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Johnson, S., M. H. Samore, K. A. Farrow, G. E. Killgore, F. C. Tenover, D. Lyras, J. I. Rood, P. DeGirolami, A. L. Baltch, M. E. Rafferty, S. M. Pear, and D. N. Gerding. 1999. Epidemics of diarrhea caused by a clindamycin-resistant strain of Clostridium difficile in four hospitals. N. Engl. J. Med. 341:1645-1651. [DOI] [PubMed] [Google Scholar]
- 93.Joseph, R., D. Demeyer, D. Vanrenterghem, R. van den Berg, E. J. Kuijper, and M. Delmee. 2005. First isolation of Clostridium difficile PCR ribotype 027, toxinotype III in Belgium. Euro Surveill. 10(10):E051020.4. http://www.eurosurveillance.org/ew/2005/051020.asp#4. [DOI] [PubMed] [Google Scholar]
- 94.Kaier, K., C. Hagist, U. Frank, A. Conrad, and E. Meyer. 2009. Two time-series analyses of the impact of antibiotic consumption and alcohol-based hand disinfection on the incidences of nosocomial methicillin-resistant Staphylococcus aureus infection and Clostridium difficile infection. Infect. Control Hosp. Epidemiol. 30:346-353. [DOI] [PubMed] [Google Scholar]
- 95.Kallen, A. J., A. Thompson, P. Ristaino, L. Chapman, A. Nicholson, B. T. Sim, F. Lessa, U. Sharapov, E. Fadden, R. Boehler, C. Gould, B. Limbago, D. Blythe, and L. C. McDonald. 2009. Complete restriction of fluoroquinolone use to control an outbreak of Clostridium difficile infection at a community hospital. Infect. Control Hosp. Epidemiol. 30:264-272. [DOI] [PubMed] [Google Scholar]
- 96.Karlström, O., B. Fryklund, K. Tullus, and L. G. Burman. 1998. A prospective nationwide study of Clostridium difficile-associated diarrhea in Sweden. The Swedish C. difficile Study Group. Clin. Infect. Dis. 26:141-145. [DOI] [PubMed] [Google Scholar]
- 97.Kato, H., Y. Ito, R. J. van den Berg, E. J. Kuijper, and Y. Arakawa. 2007. First isolation of Clostridium difficile 027 in Japan. Euro Surveill. 12(1):E070111.3. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=3110. [DOI] [PubMed] [Google Scholar]
- 98.Kazakova, S. V., K. Ware, B. Baughman, O. Bilukha, A. Paradis, S. Sears, A. Thompson, B. Jensen, L. Wiggs, J. Bessette, J. Martin, J. Clukey, K. Gensheimer, G. Killgore, and L. C. McDonald. 2006. A hospital outbreak of diarrhea due to an emerging epidemic strain of Clostridium difficile. Arch. Intern. Med. 166:2518-2524. [DOI] [PubMed] [Google Scholar]
- 99.Keel, K., J. S. Brazier, K. W. Post, S. Weese, and J. G. Songer. 2007. Prevalence of PCR ribotypes among Clostridium difficile isolates from pigs, calves, and other species. J. Clin. Microbiol. 45:1963-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khan, R., and J. Cheesbrough. 2003. Impact of changes in antibiotic policy on Clostridium difficile-associated diarrhoea (CDAD) over a five-year period in a district general hospital. J. Hosp. Infect. 54:104-108. [DOI] [PubMed] [Google Scholar]
- 101.Killgore, G., A. Thompson, S. Johnson, J. Brazier, E. Kuijper, J. Pepin, E. H. Frost, P. Savelkoul, B. Nicholson, R. J. van den Berg, H. Kato, S. P. Sambol, W. Zukowski, C. Woods, B. Limbago, D. N. Gerding, and L. C. McDonald. 2008. Comparison of seven techniques for typing international epidemic strains of Clostridium difficile: restriction endonuclease analysis, pulsed-field gel electrophoresis, PCR-ribotyping, multilocus sequence typing, multilocus variable-number tandem-repeat analysis, amplified fragment length polymorphism, and surface layer protein A gene sequence typing. J. Clin. Microbiol. 46:431-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim, H., T. V. Riley, M. Kim, C. K. Kim, D. Yong, K. Lee, Y. Chong, and J. W. Park. 2008. Increasing prevalence of toxin A-negative, toxin B-positive isolates of Clostridium difficile in Korea: impact on laboratory diagnosis. J. Clin. Microbiol. 46:1116-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim, J., S. A. Smathers, P. Prasad, K. H. Leckerman, S. Coffin, and T. Zaoutis. 2008. Epidemiological features of Clostridium difficile-associated disease among inpatients at children's hospitals in the United States, 2001-2006. Pediatrics 122:1266-1270. [DOI] [PubMed] [Google Scholar]
- 104.King, S. 2004. Provision of alcohol hand rub at the hospital bedside: a case study. J. Hosp. Infect. 56:S10-S12. [DOI] [PubMed] [Google Scholar]
- 105.Klein, E. J., D. R. Boster, J. R. Stapp, J. G. Wells, X. Qin, C. R. Clausen, D. L. Swerdlow, C. R. Braden, and P. I. Tarr. 2006. Diarrhea etiology in a children's hospital emergency department: a prospective cohort study. Clin. Infect. Dis. 43:807-813. [DOI] [PubMed] [Google Scholar]
- 106.Komatsu, M., H. Kato, M. Aihara, K. Shimakawa, M. Iwasaki, Y. Nagasaka, S. Fukuda, S. Matsuo, Y. Arakawa, M. Watanabe, and Y. Iwatani. 2003. High frequency of antibiotic-associated diarrhea due to toxin A-negative, toxin B-positive Clostridium difficile in a hospital in Japan and risk factors for infection. Eur. J. Clin. Microbiol. Infect. Dis. 22:525-529. [DOI] [PubMed] [Google Scholar]
- 107.Krook, A., G. Jarnerot, and D. Danielsson. 1981. Clinical effect of metronidazole and sulfasalazine on Crohn's disease in relation to changes in the fecal flora. Scand. J. Gastroenterol. 16:569-575. [DOI] [PubMed] [Google Scholar]
- 108.Kuijper, E. J., R. J. van den Berg, S. Debast, C. E. Visser, D. Veenendaal, A. Troelstra, T. van der Kooi, S. van den Hof, and D. W. Notermans. 2006. Clostridium difficile ribotype 027, toxinotype III in the Netherlands. Emerg. Infect. Dis. 12:827-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuijper, E. J., B. Coignard, and P. Tüll. 2006. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin. Microbiol. Infect. 12(Suppl. 6):2-18. [DOI] [PubMed] [Google Scholar]
- 110.Kuijper, E. J., B. Coignard, J. S. Brazier, C. Suetens, D. Drudy, C. Wiuff, H. Pituch, P. Reichert, F. Schneider, A. F. Widmer, K. E. Olsen, F. Allerberger, D. W. Notermans, F. Barbut, M. Delmée, M. Wilcox, A. Pearson, B. Patel, D. J. Brown, R. Frei, T. Akerlund, I. R. Poxton, and P. Tüll. 2007. Update of Clostridium difficile-associated disease due to PCR ribotype 027 in Europe. Euro Surveill. 12(6):E1-E2. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=7. [DOI] [PubMed] [Google Scholar]
- 111.Kuijper, E. J., F. Barbut, J. S. Brazier, N. Kleinkauf, T. Eckmanns, M. L. Lambert, D. Drudy, F. Fitzpatrick, C. Wiuff, D. J. Brown, J. E. Coia, H. Pituch, P. Reichert, J. Even, J. Mossong, A. F. Widmer, K. E. Olsen, F. Allerberger, D. W. Notermans, M. Delmée, B. Coignard, M. Wilcox, B. Patel, R. Frei, E. Nagy, E. Bouza, M. Marin, T. Akerlund, A. Virolainen-Julkunen, O. Lyytikäinen, S. Kotila, A. Ingebretsen, B. Smyth, P. Rooney, I. R. Poxton, and D. L. Monnet. 2008. Update of Clostridium difficile infection due to PCR ribotype 027 in Europe. Euro Surveill. 13(31):pii=18942. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=18942.
- 112.Kutty, P. K., S. R. Benoit, C. W. Woods, A. C. Sena, S. Naggie, J. Frederick, J. Engemann, S. Evans, B. C. Pien, S. N. Banerjee, J. Engel, and L. C. McDonald. 2008. Assessment of Clostridium difficile-associated disease surveillance definitions, North Carolina, 2005. Infect. Control Hosp. Epidemiol. 29:197-202. [DOI] [PubMed] [Google Scholar]
- 113.Kyne, L., C. Merry, B. O'Connell, C. Keane, and D. O'Neill. 1998. Community-acquired Clostridium difficile infection. J. Infect. 6:287-288. [DOI] [PubMed] [Google Scholar]
- 114.Labbé, A. C., L. Poirier, D. MacCannell, T. Louie, M. Savoie, C. Béliveau, M. Laverdière, and J. Pépin. 2008. Clostridium difficile infections in a Canadian tertiary care hospital before and during a regional epidemic associated with the BI/NAP1/027 strain. Antimicrob. Agents Chemother. 52:3180-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lambert, M.-L., J. Van Broeck, C. Fontaine, C. Pulincks, V. Avesani, and M. Delmée. 2009. Clinical characteristics of infections with Clostridium difficile ribotype 027 versus other ribotypes: data from prospective surveillance in Belgium, abstr. P2038. Abstr. 19th Eur. Conf. Clin. Microbiol. Infect. Dis.
- 116.Larson, H. E., F. E. Barclay, P. Honour, and I. D. Hill. 1982. Epidemiology of Clostridium difficile in infants. J. Infect. Dis. 146:727-733. [DOI] [PubMed] [Google Scholar]
- 117.Lawley, T. D., N. J. Croucher, L. Yu, S. Clare, M. Sebaihia, D. Goulding, D. J. Pickard, J. Parkhill, J. Choudhary, and G. Dougan. 2009. Proteomic and genomic characterization of highly infectious Clostridium difficile 630 spores. J. Bacteriol. 191:5377-5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leischner, J., S. Johnson, S. P. Sambol, J. P. Parada, and D. N. Gerding. 2005. Effect of alcohol hand gels and chlorhexidine handwash in removing spores of Clostridium difficile from hands, abstr. LB-29. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., Washington, DC.
- 119.Leonard, J., J. K. Marshall, and P. Moayyedi. 2007. Systematic review of the risk of enteric infection in patients taking acid suppression. Am. J. Gastroenterol. 102:2047-2056. [DOI] [PubMed] [Google Scholar]
- 120.Levy, D. G., A. Stergachis, L. V. McFarland, K. Van Vorst, D. J. Graham, E. S. Johnson, B. J. Park, D. Shatin, J. C. Clouse, and G. W. Elmer. 2000. Antibiotics and Clostridium difficile diarrhea in the ambulatory care setting. Clin. Ther. 22:91-102. [DOI] [PubMed] [Google Scholar]
- 121.Loo, V. G., L. Poirier, M. A. Miller, M. Oughton, M. D. Libman, S. Michaud, A. M. Bourgault, T. Nguyen, C. Frenette, M. Kelly, A. Vibien, P. Brassard, S. Fenn, K. Dewar, T. J. Hudson, R. Horn, P. René, Y. Monczak, and A. Dascal. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 353:2442-2449. [DOI] [PubMed] [Google Scholar]
- 122.Lowe, D. O., M. M. Mamdani, A. Kopp, D. E. Low, and D. N. Juurlink. 2006. Proton pump inhibitors and hospitalization for Clostridium difficile-associated disease: a population-based study. Clin. Infect. Dis. 43:1272-1276. [DOI] [PubMed] [Google Scholar]
- 123.Lyras, D., J. R. O'Connor, P. M. Howarth, S. P. Sambol, G. P. Carter, T. Phumoonna, R. Poon, V. Adams, G. Vedantam, S. Johnson, D. N. Gerding, and J. I. Rood. 2009. Toxin B is essential for virulence of Clostridium difficile. Nature 458:1176-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lyytikäinen, O., S. Mentula, E. Kononen, S. Kotila, E. Tarkka, and V. J. Anttila. 2007. First isolation of Clostridium difficile PCR ribotype 027 in Finland. Euro Surveill. 12(11):E071108.2. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=3303. [DOI] [PubMed] [Google Scholar]
- 125.Lyytikäinen, O., H. Turunen, R. Sund, M. Rasinperä, E. Könönen, P. Ruutu, and I. Keskimäki. 2009. Hospitalizations and deaths associated with Clostridium difficile infection, Finland, 1996-2004. Emerg. Infect. Dis. 15:761-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lyytikäinen, O., T. Möttönen, S. Kotila, S. Ibrahem, and A. Virolainen-Julkunen. 2009. First results of hospital-based surveillance of Clostridium difficile-associated infections in Finnish acute care hospitals, abstr. P1434. Abstr. 19th Eur. Congr. Clin. Microbiol. Infect. Dis., Helsinki, Finland.
- 127.MacCannell, D. R., T. J. Louie, D. B. Gregson, M. Laverdiere, A. C. Labbe, F. Laing, and S. Henwick. 2006. Molecular analysis of Clostridium difficile PCR ribotype 027 isolates from Eastern and Western Canada. J. Clin. Microbiol. 44:2147-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Martin, H., B. Willey, D. E. Low, H. R. Staempfli, A. McGeer, P. Boerlin, M. Mulvey, and J. S. Weese. 2008. Characterization of Clostridium difficile isolated from patients in Ontario, Canada, from 2004 to 2006. J. Clin. Microbiol. 46:2999-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McCusker, M. E., A. D. Harris, E. Perencevich, and M.-C. Roghmann. 2003. Fluoroquinolone use and Clostridium difficile-associated diarrhea. Emerg. Infect. Dis. 9:730-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McDonald, L. C., G. E. Killgore, A. Thompson, R. C. Owens, Jr., S. V. Kazakova, S. P. Sambol, S. Johnson, and D. N. Gerding. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353:2433-2441. [DOI] [PubMed] [Google Scholar]
- 131.McDonald, L. C., M. Owings, and D. B. Jernigan. 2006. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996-2003. Emerg. Infect. Dis. 12:409-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McDonald, L. C., B. Coignard, E. Dubberke, X. Song, T. Horan, P. K. Kutty, and the Ad Hoc Clostridium difficile Surveillance Working Group. 2007. Recommendations for surveillance of Clostridium difficile-associated disease. Infect. Control Hosp. Epidemiol. 28:140-145. [DOI] [PubMed] [Google Scholar]
- 133.McFarland, L. V., C. M. Surawicz, and W. E. Stamm. 1990. Risk factors for Clostridium difficile carriage and C. difficile-associated diarrhea in a cohort of hospitalized patients. J. Infect. Dis. 162:678-684. [DOI] [PubMed] [Google Scholar]
- 134.McFarland, L. V., J. E. Claridge, H. W. Beneda, and G. J. Raugi. 2007. Fluoroquinolone use and risk factors for Clostridium difficile associated disease within a Veterans Administration health care system. Clin. Infect. Dis. 45:1141-1151. [DOI] [PubMed] [Google Scholar]
- 135.McFarland, L. V. 2009. Renewed interest in a difficult disease: Clostridium difficile infections—epidemiology and current treatment strategies. Curr. Opin. Gastroenterol. 25:24-35. [DOI] [PubMed] [Google Scholar]
- 136.McNulty, C., M. Logan, I. P. Donald, D. Ennis, D. Taylor, R. N. Baldwin, M. Bannerjee, and K. A. Cartwright. 1997. Successful control of Clostridium difficile infection in an elderly care unit through use of a restrictive antibiotic policy. J. Antimicrob. Chemother. 40:707-711. [DOI] [PubMed] [Google Scholar]
- 137.Morgan, O. W., B. Rodrigues, T. Elston, N. Q. Verlander, D. F. Brown, J. Brazier, and M. Reacher. 2008. Clinical severity of Clostridium difficile PCR ribotype 027: a case-case study. PLoS One 19:e1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Musher, D. M., S. Aslam, N. Logan, S. Nallacheru, I. Bhaila, F. Borchert, and R. J. Hamill. 2005. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin. Infect. Dis. 40:1586-1590. [DOI] [PubMed] [Google Scholar]
- 139.Muto, C. A., M. Pokrywka, K. Shutt, A. B. Mendelsohn, K. Nouri, K. Posey, T. Roberts, K. Croyle, S. Krystofiak, S. Patel-Brown, A. W. Pasculle, D. L. Paterson, M. Saul, and L. H. Harrison. 2005. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect. Control Hosp. Epidemiol. 26:273-280. [DOI] [PubMed] [Google Scholar]
- 140.Muto, C. A., M. K. Blank, J. W. Marsh, E. N. Vergis, M. M. O'Leary, K. A. Shutt, A. W. Pasculle, M. Pokrywka, J. G. Garcia, K. Posey, T. L. Roberts, B. A. Potoski, G. E. Blank, R. L. Simmons, P. Veldkamp, L. H. Harrison, and D. L. Paterson. 2007. Control of an outbreak of infection with the hypervirulent Clostridium difficile BI strain in a university hospital using a comprehensive “bundle” approach. Clin. Infect. Dis. 45:1266-1273. [DOI] [PubMed] [Google Scholar]
- 141.Nasereddin, L. M., F. G. Bakri, and A. A. Shehabi. 25 August 2009, posting date. Clostridium difficile infections among Jordanian adult hospitalized patients. Am. J. Infect. Control [Epub ahead of print.] doi: 10.1016/j.ajic.2009.05.001. [DOI] [PubMed]
- 142.Norén, T., T. Åkerlund, E. Bäck, L. Sjöberg, I. Persson, I. Alriksson, and L. G. Burman. 2004. Molecular epidemiology of hospital-associated and community-acquired Clostridium difficile infection in a Swedish county. J. Clin. Microbiol. 42:3635-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.O'Connor, J. R., S. Johnson, and D. N. Gerding. 2009. Clostridium difficile infection caused by the epidemic B1/NAP1/027 strain. Gastroenterology 136:1913-1924. [DOI] [PubMed] [Google Scholar]
- 144.Office for National Statistics. 2009. Statistical bulletin. Deaths involving Clostridium difficile: England & Wales, 2008. Office for National Statistics, London, United Kingdom. http://www.statistics.gov.uk/statbase/Product.asp?vlnk=14782.
- 145.Oughton, M. T., V. G. Loo, N. Dendukuri, S. Fenn, and M. D. Libman. 2009. Hand hygiene with soap and water is superior to alcohol rub and antiseptic wipes for removal of Clostridium difficile. Infect. Control Hosp. Epidemiol. 30:939-944. [DOI] [PubMed] [Google Scholar]
- 146.Palmore, T. N., S. Sohn, S. F. Malak, J. Eagan, and K. A. Sepkowitz. 2005. Risk factors for acquisition of Clostridium difficile-associated diarrhea among outpatients at a cancer hospital. Infect. Control Hosp. Epidemiol. 26:680-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Paltansing, S., R. J. van den Berg, R. A. Guseinova, C. E. Visser, E. R. van der Vorm, and E. J. Kuijper. 2007. Characteristics and incidence of Clostridium difficile-associated disease, the Netherlands, 2005. Clin. Microbiol. Infect. 13:1058-1064. [DOI] [PubMed] [Google Scholar]
- 148.Pear, S. M., T. H. Williamson, K. M. Bettin, D. N. Gerding, and J. N. Galgiani. 1994. Decrease in nosocomial Clostridium difficile-associated diarrhea by restricting clindamycin use. Ann. Intern. Med. 120:272-277. [DOI] [PubMed] [Google Scholar]
- 149.Pelaez, T., L. Alcala, L. Martinez-Sanchez, P. Munoz, J. M. Garcia-Lechuz, M. Rodriguez-Creixems, and E. Bouza. 1998. Metronidazole resistance in Clostridium difficile: a new emerging problem, abstr. E-173. Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 150.Pelaez, T., L. Alcala, R. Alonso, M. Rodriguez-Creixems, J. M. Garcia-Lechuz, and E. Bouza. 2002. Reassessment of Clostridium difficile susceptibility to metronidazole and vancomycin. Antimicrob. Agents Chemother. 46:1647-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pelaez, T., E. Cercenado, L. Alcala, M. Marin, A. Martin-Lopez, J. Martinez-Alarcon, P. Catalan, M. Sanchez-Somolinos, and E. Bouza. 2008. Metronidazole resistance in Clostridium difficile is heterogeneous. J. Clin. Microbiol. 46:3028-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pépin, J., L. Valiquette, M. E. Alary, P. Villemure, A. Pelletier, K. Forget, K. Pépin, and D. Chouinard. 2004. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ 171:466-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pepin, J., L. Valiquette, and B. Cossette. 2005. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ 173:1037-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pepin, J., M. E. Alary, L. Valiquette, E. Raiche, J. Ruel, K. Fulop, D. Godin, and C. Bourassa. 2005. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin. Infect. Dis. 40:1591-1597. [DOI] [PubMed] [Google Scholar]
- 155.Pepin, J., N. Saheb, M. A. Coulombe, M. E. Alary, M. P. Corriveau, S. Authier, M. Leblanc, G. Rivard, M. Bettez, V. Primeau, M. Nguyen, C. E. Jacob, and L. Lanthier. 2005. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin. Infect. Dis. 41:1254-1260. [DOI] [PubMed] [Google Scholar]
- 156.Pittet, D., B. Allegranzi, and J. Boyce. 2009. The World Health Organization guidelines on hand hygiene in health care and their consensus recommendations. Infect. Control Hosp. Epidemiol. 30:611-622. [DOI] [PubMed] [Google Scholar]
- 157.Pituch, H., D. Bakker, E. Kuijper, P. Obuch-Woszczatyński, D. Wultańska, G. Nurzyńska, A. Bielec, E. Bar-Andziak, and M. Łuczak. 2008. First isolation of Clostridium difficile PCR-ribotype 027/toxinotype III in Poland. Pol. J. Microbiol. 57:267-268. [PubMed] [Google Scholar]
- 158.Planche, T., A. Aghaizu, R. Holliman, P. Riley, J. Poloniecki, A. Breathnach, and S. Krishna. 2008. Diagnosis of Clostridium difficile infection by toxin detection kits: a systematic review. Lancet Infect. Dis. 8:777-784. [DOI] [PubMed] [Google Scholar]
- 159.Ricciardi, R., D. A. Rothenberger, R. D. Madoff, and N. N. Baxter. 2007. Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Arch. Surg. 142:624-631. [DOI] [PubMed] [Google Scholar]
- 160.Riggs, M. M., A. K. Sethi, T. F. Zabarsky, E. C. Eckstein, R. L. P. Jump, and C. J. Donskey. 2007. Asymptomatic carriers are a potential source for transmission of epidemic and non-epidemic Clostridium difficile strains among long-term care facility residents. Clin. Infect. Dis. 45:992-998. [DOI] [PubMed] [Google Scholar]
- 161.Riley, T. V., V. Wymer, V. W. Bamford, and R. A. Bowman. 1986. Clostridium difficile in general practice and community health. J. Hyg. (Lond.) 96:13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Riley, T. V., F. Wetherall, J. Bowman, J. Mogyorosy, and C. L. Colledge. 1991. Diarrheal disease due to Clostridium difficile in general practice. Pathology 23:346-349. [DOI] [PubMed] [Google Scholar]
- 163.Riley, T. V., M. Cooper, B. Bell, and C. L. Golledge. 1995. Community-acquired Clostridium difficile-associated diarrhoea. Clin. Infect. Dis. 20(Suppl. 2):5263-5265. [DOI] [PubMed] [Google Scholar]
- 164.Riley, T. V., S. Thean, G. Hool, and C. L. Golledge. 2009. First Australian isolation of epidemic Clostridium difficile PCR ribotype 027. Med. J. Aust. 190:706-708. [DOI] [PubMed] [Google Scholar]
- 165.Rodriguez-Palacios, A., H. R. Stämpfli, T. Duffield, A. S. Peregrine, L. A. Trotz-Williams, L. G. Arroyo, J. S. Brazier, and J. S. Weese. 2006. Clostridium difficile PCR ribotypes in calves, Canada. Emerg. Infect. Dis. 12:1730-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rodriguez-Palacios, A., R. J. Reid-Smith, H. R. Staempfli, D. Daignault, N. Janecko, B. P. Avery, H. Martin, A. D. Thomspon, L. C. McDonald, B. Limbago, and J. S. Weese. 2009. Possible seasonality of Clostridium difficile in retail meat, Canada. Emerg. Infect. Dis. 15:802-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rotimi, V. O., W. Y. Jamal, E. M. Mokaddas, J. S. Brazier, M. Johny, and B. I. Duerden. 2003. Prevalent PCR ribotypes of clinical and environmental strains of Clostridium difficile isolated from intensive-therapy unit patients in Kuwait. J. Med. Microbiol. 52:705-709. [DOI] [PubMed] [Google Scholar]
- 168.Rupnik, M. 2007. Is Clostridium difficile-associated infection a potentially zoonotic and foodborne disease? Clin. Microbiol. Infect. 13:457-459. [DOI] [PubMed] [Google Scholar]
- 169.Rupnik, M., A. Widmer, O. Zimmermann, C. Eckert, and F. Barbut. 2008. Clostridium difficile 383 toxinotype V, ribotype 078, in animals and humans. J. Clin. Microbiol. 46:2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rupp, M. E., T. Fitzgerald, S. Puumala, J. R. Anderson, R. Craig, P. C. Iwen, D. Jourdan, J. Keuchel, N. Marion, D. Peterson, L. Sholtz, and V. Smith. 2008. Prospective, controlled, cross-over trial of alcohol-based hand gel in critical care units. Infect. Control Hosp. Epidemiol. 29:8-15. [DOI] [PubMed] [Google Scholar]
- 171.Sambol, S. P., F. S. Siddiqui, T. Akerlund, R. Goering, F. Tenover, S. Johnson, and D. Gerding. 2009. Variable fluoroquinolone resistance between emerging North American and European isolates of Clostridium difficile REA group DH (PCR ribotype 106), abstr. P24. Abstr. 6th ClostPath Int. Conf., Rome, Italy.
- 172.Sanchez, J. L., D. N. Gerding, M. M. Olson, and S. Johnson. 1999. Metronidazole susceptibility in Clostridium difficile isolates recovered from cases of C. difficile-associated disease treatment failures and successes. Anaerobe 5:201-204. [Google Scholar]
- 173.Sawabe, E., H. Kato, K. Osawa, T. Chida, N. Tojo, Y. Arakawa, and N. Okamura. 2007. Molecular analysis of Clostridium difficile at a university teaching hospital in Japan: a shift in the predominant type over a five-year period. Eur. J. Clin. Microbiol. Infect. Dis. 26:695-703. [DOI] [PubMed] [Google Scholar]
- 174.Schwann, C., B. Stecher, T. Tzivelekidis, M. van Ham, M. Rohde, W.-F. Hardt, J. Wehland, and K. Aktories. 2009. Clostridium difficile CDT induces formation of microtubule-based protrusions and increases adherence of bacteria. PLoS Pathog. 5:e1000626. doi: 10.1371/journal.ppar.100626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shin, B. M., E. Y. Kuak, H. M. Yoo, E. C. Kim, K. Lee, J. O. Kang, D. H. Whang, and J. H. Shin. 2008. Multicentre study of the prevalence of toxigenic Clostridium difficile in Korea: results of a retrospective study 2000-2005. J. Med. Microbiol. 57:697-701. [DOI] [PubMed] [Google Scholar]
- 176.Shin, B. M., E. Y. Kuak, S. J. Yoo, W. C. Shin, and H. M. Yoo. 2008. Emerging toxin A−B+ variant strain of Clostridium difficile responsible for pseudomembranous colitis at a tertiary care hospital in Korea. Diagn. Microbiol. Infect. Dis. 60:333-337. [DOI] [PubMed] [Google Scholar]
- 177.Smith, A. 2005. Outbreak of Clostridium difficile infection in an English hospital linked to hypertoxin-producing strains in Canada and the US. Euro Surveill. 10(6):E050630.2. http://www.eurosurveillance.org/ew/2005/050630asp#2. [DOI] [PubMed] [Google Scholar]
- 178.Snydman, D. R., N. V. Jacobus, and L. A. McDermott. 2009. In vitro activity of a ceftaroline versus a broad spectrum of recent clinical anaerobic isolates, abstr. E-203. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Fransisco, CA. [DOI] [PMC free article] [PubMed]
- 179.Solomon, K., A. Martin, C. O'Donahue, S. Murray, S. Fanning, and L. Kyne. 2009. Reduced sensitivity of Clostridium difficile to metronidazole and implications for treatment, abstr. K-2088. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA.
- 180.Sonenshein, A. L. 2005. codY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr. Opin. Microbiol. 8:203-207. [DOI] [PubMed] [Google Scholar]
- 181.Songer, J. G., H. T. Trinh, S. M. Dial, J. S. Brazier, and R. D. Glock. 2009. Equine colitis X associated with infection by Clostridium difficile NAP1/027. J. Vet. Diagn. Invest. 21:377-380. [DOI] [PubMed] [Google Scholar]
- 182.Songer, J. G., H. T. Trinh, G. E. Killgore, A. D. Thompson, L. C. McDonald, and B. M. Limbago. 2009. Clostridium difficile in retail meat products, USA, 2007. Emerg. Infect. Dis. 15:819-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Spigaglia, P., F. Barbanti, T. Louie, F. Barbut, and P. Mastrantonio. 2009. Molecular analysis of the gyrA and gyrB quinolone resistance-determining regions of fluoroquinolone-resistant Clostridium difficile mutants selected in vitro. Antimicrob. Agents Chemother. 53:2463-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Stabler, R. A., M. He, L. Dawson, M. Martin, E. Valiente, C. Corton, T. D. Lawley, M. Sebaihia, M. A. Quail, G. Rose, D. N. Gerding, M. Gibert, M. R. Popoff, J. Parkhill, G. Dougan, and B. W. Wren. 2009. Comparative genome and phenotypic analysis of Clostridium difficile 027 strains provides insight into the evolution of a hypervirulent bacterium. Genome Biol. 10:R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Stone, S., C. Fuller, R. Slade, J. Savage, A. Charlett, B. Cookson, B. S. Cooper, G. Duckworth, M. Murray, A. Hayward, A. Jeanes, J. Roberts, L. Teare, J. McAteer, and S. Michie. 2008. The success and effectiveness of the worlds first national clean your hands campaign in England and Wales 2004-2008, abstr. O-140. Abstr. 18th Eur. Congr. Clin. Microbiol. Infect. Dis., Barcelona, Spain.
- 186.Sundram, F., A. Guyot, I. Carboo, S. Green, M. Lilaonitkul, and A. Scourfield. 2009. Clostridium difficile ribotypes 027 and 106: clinical outcomes and risk factors. J. Hosp. Infect. 72:111-118. [DOI] [PubMed] [Google Scholar]
- 187.Svenungsson, B., L. G. Burman, K. Jalakas-Pörnull, A. Lagergren, J. Struwe, and T. Akerlund. 2003. Epidemiology and molecular characterization of Clostridium difficile strains from patients with diarrhea: low disease incidence and evidence of limited cross-infection in a Swedish teaching hospital. J. Clin. Microbiol. 41:4021-4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tae, C. H., S. A. Jung, H. J. Song, S. E. Kim, H. J. Choi, M. Lee, Y. Hwang, H. Kim, and K. Lee. 2009. The first case of antibiotic-associated colitis by Clostridium difficile PCR ribotype 027 in Korea. J. Korean Med. Sci. 24:520-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tal, S., A. Gurevich, V. Guller, I. Gurevich, D. Berger, and S. Levi. 2002. Risk factors for recurrence for Clostridium difficile-associated diarrhea in the elderly. Scand. J. Infect. Dis. 34594-34597. [DOI] [PubMed]
- 190.Tan, E. T., C. A. Robertson, S. Brynildsen, E. Bresnitz, C. Tan, and C. McDonald. 2007. Clostridium difficile-associated disease in New Jersey hospitals, 2000-2004. Emerg. Infect. Dis. 13:498-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Terhes, G., E. Urbán, M. Konkoly-Thege, E. Székely, J. S. Brazier, E. J. Kuijper, and E. Nagy. 2009. First isolation of Clostridium difficile PCR ribotype 027 from a patient with severe persistent diarrhoea in Hungary. Clin. Microbiol. Infect. 15:885-886. [DOI] [PubMed] [Google Scholar]
- 192.Thibault, A., M. A. Miller, and C. Gaese. 1991. Risk factors for the development of Clostridium difficile-associated diarrhea during a hospital outbreak. Infect. Control Hosp. Epidemiol. 12:345-348. [DOI] [PubMed] [Google Scholar]
- 193.Thomas, C., M. Stevenson, D. J. Williamson, and T. V. Riley. 2002. Clostridium difficile-associated diarrhea: epidemiological data from Western Australia associated with a modified antibiotic policy. Clin. Infect. Dis. 35:1457-1462. [DOI] [PubMed] [Google Scholar]
- 194.Thomas, C., M. Stevenson, and T. V. Riley. 2003. Antibiotics and hospital-acquired Clostridium difficile-associated diarrhoea: a systematic review. J. Antimicrob. Chemother. 51:1339-1350. [DOI] [PubMed] [Google Scholar]
- 195.Valiquette, L., B. Cossette, M. P. Garant, H. Diab, and J. Pepin. 2007. Impact of a reduction in the use of high-risk antibiotics on the course of an epidemic of Clostridium difficile-associated disease caused by the hypervirulent NAP1/027 strain. Clin. Infect. Dis. 45:S112-S121. [DOI] [PubMed] [Google Scholar]
- 196.van den Berg, R. J., E. C. Claas, D. H. Oyib, C. H. Klaassen, L. Dijkshoorn, J. S. Brazier, and E. J. Kuijper. 2004. Characterization of toxin A-negative, toxin B-positive Clostridium difficile isolates from outbreaks in different countries by amplified fragment length polymorphism and PCR ribotyping. J. Clin. Microbiol. 42:1035-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.van den Berg, R. J., I. Schapp, K. E. Templeton, C. H. W. Klaassen, and E. J. Kuijper. 2007. Typing and subtyping of Clostridium difficile isolates using multiple-locus variable-number tandem-repeat analysis. J. Clin. Microbiol. 45:1024-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.van der Kooi, T. I. I., M. Koningstein, A. Lindemans, D. W. Notermans, E. J. Kuijper, R. van den Berg, H. Boshuizen, P. M. G. Filius, and S. van den Hof. 2008. Antibiotic use and other risk factors at hospital level for outbreaks with Clostridium difficile PCR ribotype 027. J. Med. Microbiol. 57:709-716. [DOI] [PubMed] [Google Scholar]
- 199.van Steenbergen, J., S. Debast, E. van Kregten, R. van den Berg, D. Notermans, and E. J. Kuijper. 2005. Isolation of Clostridium difficile ribotype 027, toxinotype III in the Netherlands after increase in C. difficile-associated diarrhoea. Euro Surveill. 10(7):E050714.1. http://www.eurosurveillance.org/ew/2005/050714.asp#1. [PubMed] [Google Scholar]
- 200.Vernaz, N., H. Sax, D. Pittet, P. Bonnabry, J. Schrenzel, and S. Harbarth. 2008. Temporal effects of antibiotic use and hand rub consumption on the incidence of MRSA and Clostridium difficile. J. Antimicrob. Chemother. 62:601-607. [DOI] [PubMed] [Google Scholar]
- 201.Von Abercron, S. M., F. Karlsson, G. T. Wigh, M. Wierup, and K. Krovacek. 2009. Low occurrence of Clostridium difficile in retail ground meat in Sweden. J. Food Prot. 72:1732-1734. [DOI] [PubMed] [Google Scholar]
- 202.Vonberg, R. P., E. J. Kuijper, M. H. Wilcox, F. Barbut, P. Tüll, P. Gastmeier, European C difficile-Infection Control Group, European Centre for Disease Prevention and Control, P. J. van den Broek, A. Colville, B. Coignard, T. Daha, S. Debast, B. I. Duerden, S. van den Hof, T. van der Kooi, H. J. Maarleveld, E. Nagy, D. W. Notermans, J. O'Driscoll, B. Patel, S. Stone, and C. Wiuff. 2008. Infection control measures to limit the spread of Clostridium difficile. Clin. Microbiol. Infect. 14(Suppl. 5):2-20. [DOI] [PubMed] [Google Scholar]
- 203.Warny, M., J. Pepin, A. Fang, G. Killgore, A. Thompson, J. Brazier, E. Frost, and L. C. McDonald. 2005. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 366:1079-1084. [DOI] [PubMed] [Google Scholar]
- 204.Weese, J. S., B. P. Avery, J. Rousseau, and R. J. Reid-Smith. 2009. Detection and enumeration of Clostridium difficile spores in retail beef and pork. Appl. Environ. Microbiol. 75:5009-5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Weese, J. S., R. Finlay, R. R. Reid-Smith, N. Janecko, and J. Rousseau. 2009. Evaluation of Clostridium difficile in dogs and the household environment. Epidemiol. Infect. [Epub ahead of print.] doi: 10.1017/S0950268809991312. [DOI] [PubMed]
- 206.Weil, H. P., U. Fischer-Brügge, C. Harmanus, F. Mattner, P. Gastmeier, and E. J. Kuijper. 2007. High incidence of Clostridium difficile-associated diarrhea with a community onset in a hyperendemic region in Germany, abstr. O329. Abstr. 17th Eur. Congr. Clin. Microbiol. Infect. Dis./25th Int. Conf. Chemother.
- 207.Weiss, B., N. Kleinkauf, T. Eckmanns, M. an der Heiden, M. Neumann, H. Michels, and A. Jansen. 2009. Risk factors related to a hospital-associated cluster of Clostridium difficile PCR ribotype 027 infections in Germany during 2007. Infect. Control Hosp. Epidemiol. 30:282-284. [DOI] [PubMed] [Google Scholar]
- 208.Weiss, K. 2006. Poor infection control, not fluoroquinolones, likely to be primary cause of Clostridium difficile-associated diarrhoea outbreaks in Quebec. Clin. Infect. Dis. 42:725-727. [DOI] [PubMed] [Google Scholar]
- 209.Weiss, K., A. Boisvert, M. Chagnon, C. Duchesne, S. Habash, Y. Lepage, J. Letourneau, J. Raty, and M. Savoie. 2009. Clostridium difficile rates from 2002 to 2007: impact of a multi-pronged intervention strategy to control and outbreak situation. Infect. Control Hosp. Epidemiol. 30:156-162. [DOI] [PubMed] [Google Scholar]
- 210.Wheeler, J. G., D. Sethi, J. M. Cowden, P. G. Wall, L. C. Rodrigues, D. S. Tompkins, M. J. Hudson, and P. J. Roderick. 1999. Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to national surveillance. BMJ 318:1046-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wilcox, M. H., and W. N. Fawley. 2000. Hospital disinfectants and spore formation by Clostridium difficile. Lancet 356:1324. [DOI] [PubMed] [Google Scholar]
- 212.Wilcox, M. H., J. Freeman, W. N. Fawley, S. MacKinlay, A. Brown, K. Donaldson, and O. Corrado. 2004. Long-term surveillance of cefotaxime and piperacillin-tazobactam prescribing and incidence of Clostridium difficile diarrhoea. J. Antimicrob. Chemother. 54:168-172. [DOI] [PubMed] [Google Scholar]
- 213.Wilcox, M. H., and J. Freeman. 2006. Epidemic Clostridium difficile. N. Engl. J. Med. 354:1199-1203. [PubMed] [Google Scholar]
- 214.Wilcox, M. H., L. Mooney, R. Bendall, C. D. Settle, and W. N. Fawley. 2008. A case-control study of community-associated Clostridium difficile infection. J. Antimicrob. Chemother. 62:388-396. [DOI] [PubMed] [Google Scholar]
- 215.Wistrom, J., S. R. Norrby, E. B. Myhre, S. Eriksson, G. Granstrom, L. Lagergren, G. Englund, C. E. Nord, and B. Svenungsson. 2001. Frequency of antibiotic-associated diarrhoea in 2462 antibiotic-treated hospitalized patients: a prospective study. J. Antimicrob. Chemother. 47:43-50. [DOI] [PubMed] [Google Scholar]
- 216.Wong, S. S., P. C. Woo, W. K. Luk, and K. Y. Yuen. 1999. Susceptibility testing of Clostridium difficile against metronidazole and vancomycin by disk diffusion and Etest. Diagn. Microbiol. Infect. Dis. 34:1-6. [DOI] [PubMed] [Google Scholar]
- 217.Yip, C., M. Loeb, S. Salama, L. Moss, and J. Olde. 2001. Quinolone use as a risk factor for nosocomial Clostridium difficile-associated diarrhea. Infect. Control Hosp. Epidemiol. 22:572-575. [DOI] [PubMed] [Google Scholar]
- 218.Young, G. P., P. B. Ward, N. Bayley, D. Gordon, G. Higgins, J. A. Trapani, M. I. McDonald, J. Labrooy, and R. Hecker. 1985. Antibiotic-associated colitis due to Clostridium difficile: double-blind comparison of vancomycin with bacitracin. Gastroenterology 89:1038-1045. [DOI] [PubMed] [Google Scholar]
- 219.Zilberberg, M. D., A. F. Shorr, and M. H. Kollef. 2008. Increase in adult Clostridium difficile related hospitalizations and case fatality in the US, 2000-2005. Emerg. Infect. Dis. 14:929-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zilberberg, M. D. 2009. Clostridium difficile-related hospitalizations among US adults, 2006. Emerg. Infect. Dis. 15:122-124. [DOI] [PMC free article] [PubMed] [Google Scholar]