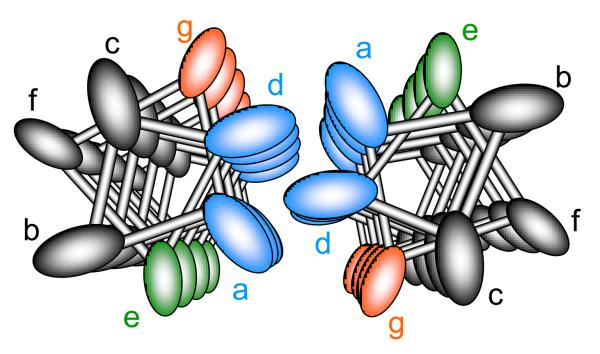
Figure 1.
Helical relation of amino acid positions of tropomyosin's coiled coil as viewed from an axial perspective. Hydrophobic residues at position a and d stabilize interactions between α helices, where positions g and e are typically charged amino acids stabilizing tropomyosin through ionic interactions.