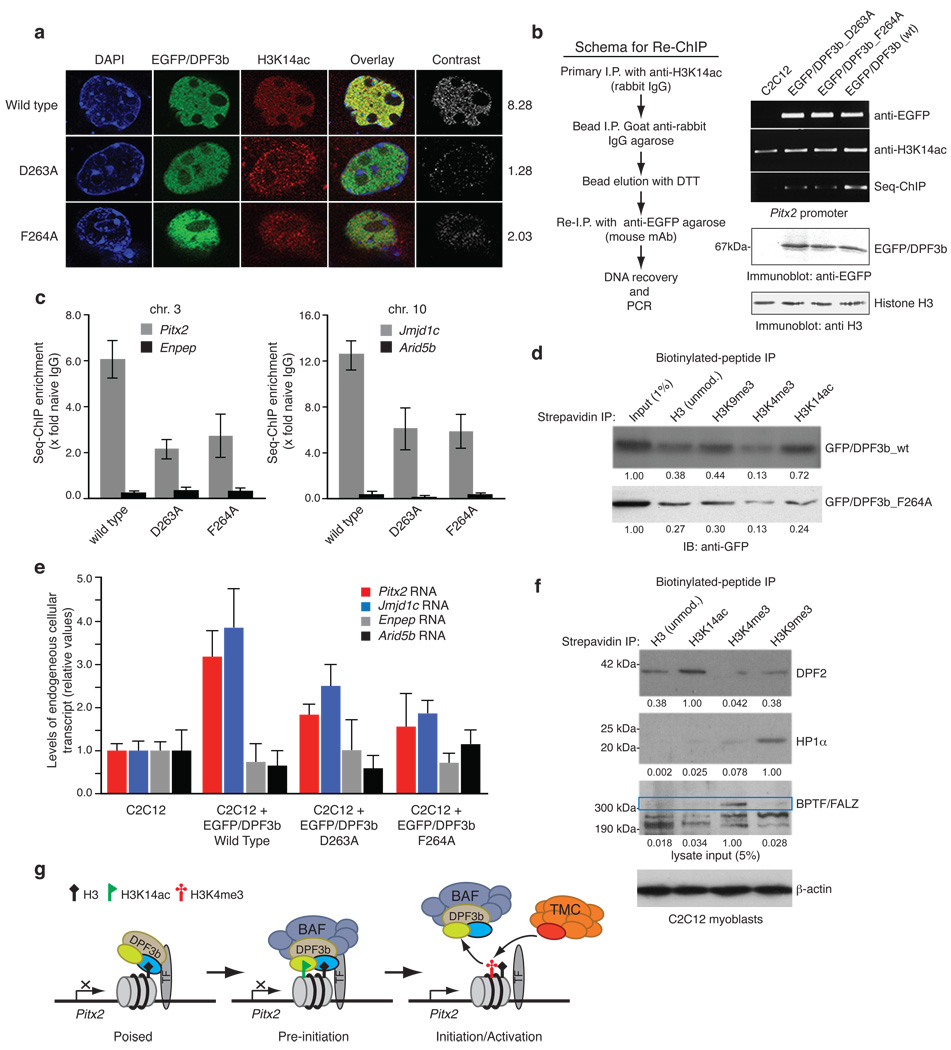
Figure 4. Acetylation and methylation modulated histone H3 binding by DPF3b PHD12 is important for gene transcriptional activation.
(a) Co-localization of EGFP-DPF3b PHD12, or its acetyl-lysine binding deficient mutants, and H3K14ac in the C2C12 cell nucleus visualized by confocal fluorescence imaging. Calculated mean normalized total signal intensity value for each DPF3b is listed on the right (Supplementary Table 4). (b) Sequential chromatin immunoprecipitation (re-ChIP) assay (scheme, left) used to assess EGFP-DPF3b or its PHD12 mutants in interactions with H3K14ac at the mouse Pitx2 promoter site (right). (c) Quantitation of DNA recovered for Pitx2 and Jmjd1c promoters by qPCR following enrichment by immunoprecipitations with anti-sera against H3K14ac and seq-ChIP of EGFP/DPF3b with anti-EGFP agarose. Two non-DPF3b target genes Enpep in chromosome 3 and Arid5b in chromosome 10 were used in qChIP as negative controls to Pitx2 and Jmjd1c, respectively. (d) Assessing wild type DPF3b and mutant F264A binding to C-terminal biotinylated histone H3 peptides of different modifications in a peptide pull-down assay. Immunoblots depict H3 peptides binding to DPF3b from nuclear extracts of C2C12 cells transfected with the corresponding GFP/DPF3b plasmid. Signals in the immunoblots were quantified using NIH’s ImageJ software, and normalized to the input (1%) as 1.00. (e) Relative transcript levels of endogenous Pitx2 and Jmjd1c in C2C12 cells 2 days following transfections with wild type and mutant DPF3b cDNAs as indicated. (f) Evaluation of endogenous DPF2, HP1α and BPTF/FALZ in nuclear extracts of C2C12 cells binding to histone H3 peptides of different modifications. A single filter was used to determine retention of specific endogenous protein for each of the H3 peptides as shown. Separately, 5% of overall input for the peptide pull-down was monitored with β-actin. Blots were scanned and densitometry measurements were performed as described in d. (g) Schematic diagram illustrating modulation of human DPF3b PHD12 binding to histone H3 by site-specific lysine acetylation and methylation during poised, pre-initiation and initiation/activation stages of gene transcription. PHD1 and PHD2 of DPF3b are color-coded in yellow and blue, respectively. TF stands for a gene specific transcription factor, and TMC (orange) represents the transcriptional machinery complex.