Figure 3.
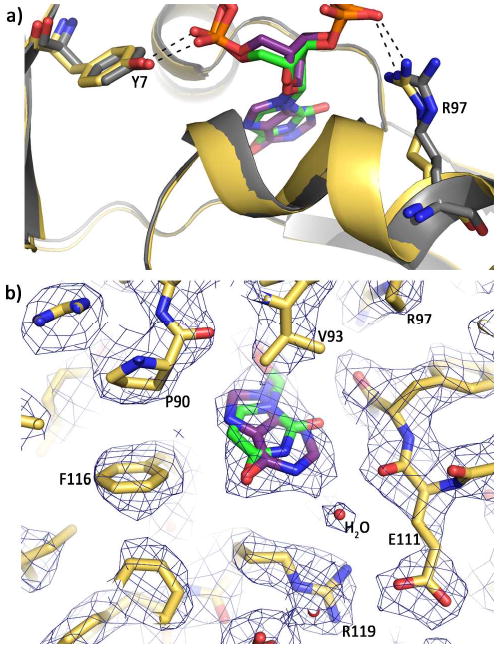
Comparison of hypoxanthine and uracil recognition. (a) Overlay of the hypoxanthine (purple) (this publication) and uracil (green) (4) structures with Tgo-Pol. The base, sugar and phosphates of the two deaminated bases are nearly super-imposable, as are the amino-terminal domains (yellow and grey for the hypoxanthine and uracil structures respectively). Side chain overlap is also very high, as shown for Tyr 7 and Arg 97, which bind the phosphates flanking the deaminated bases. (b) Superimposition of uracil (green) onto the structure of hypoxanthine (purple) bound to Tgo-Pol. The C5-C6 edge of uracil is near Pro 90 and Phe 116 (and also Pro 36, which is not visible in this view) and the C2 of hypoxanthine is close to the main chain carbonyl oxygen of Glu 111. Such tight packing of the deaminated bases results in steric exclusion of the larger thymine (5-CH3) and guanine (2-NH2) bases. The water molecule involved in a water-mediated hydrogen bond with hypoxanthine is also shown.
