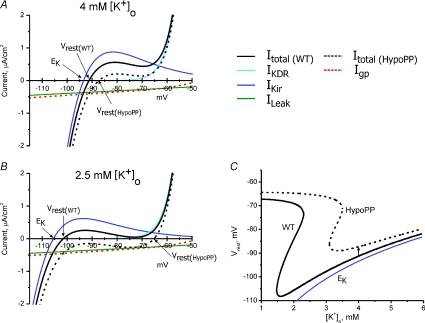
Figure 3. The gating pore current increases the susceptibility to paradoxical depolarization in low extracellular [K+].
The steady-state I–V relation for mammalian skeletal muscle was simulated by the combination of an inward rectifier K+ current, IKir, a delayed rectifier K+ current, IKDR, and a leakage current with a reversal potential of 0 mV, ILeak, as in Struyk & Cannon (2008). A, in 4 mm[K+]o the resting potential of −91.3 mV is determined primarily from the balance of an inward ILeak and outward IKir. Addition of a gating pore current, Igp, to simulate HypoPP (dashed red line), shifts the I–V relation downward (dashed black line) but results in only a small depolarization of Vrest to −87.3 mV. B, reduction of [K+]o to 2.5 mm shifts EK and IKir to more negative potentials, with a predicted hyperpolarization of Vrest to −101.6 mV in WT fibres. For HypoPP fibres, however, Vrest depolarizes to −65.3 mV (arrow) because the combination of inward currents (ILeak+Igp) exceeds the outward current from IKir. Under these conditions, which would result in paralysis from inactivation of sodium channels, Vrest is now set by the balance of IKDR and the inward currents. Because KDR channels are active only for >−55 mV, Vrest is strongly dependent on KDR gating, which results in an apparent decoupling of Vrest from EK. C, phase plot of Vrest as a function of [K+]o for simulated WT and HypoPP fibres. The inward gating pore current in HypoPP fibres causes only a small depolarization of Vrest from −91.3 mV to −87.3 mV in 4 mm[K+]o (arrow). More importantly, the catastrophic depolarization of Vrest shifts leftward from 1.5 mm[K+]o for WT to 3 mm for HypoPP. The Nernst potential for K+, EK, is shown for comparison (blue line).