Table 2.
Parameter values adjusted and optimized to match model simulations to the data from EDL muscles for all durations of anoxia
| Parameter | Definition | Reported value, EDL | Optimized value, EDL | Coefficient of variation (%) | Reported value, SOL | Optimized value, SOL | Coefficient of variation (%) |
|---|---|---|---|---|---|---|---|
| KATP | Rate constant for ATP consumption | 0.065 min−1a | 0.0828 min−1 | 7.81 | 0.12 min−1a | 0.124 min−1 | 7.39 |
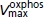 |
Vmax of oxidative phosphorylation | 3.4 × 10−3m min−1 b | 3.75 × 10−3m min−1 | 11.87 | 3.2 × 10−3m min−1b | 3.71 × 10−3m min−1 | 19.03 |
 |
Phenomenological ADP kinetic constant for oxidative phosphorylation | 4.4 × 10−5mc | 4.83 × 10−5m | – | 4.4 × 10−5mc | 4.19 × 10−5m | – |
 |
Hill coefficient of ADP for oxidative phosphorylation | >2c | 2.27 | – | >2c | 2.22 | – |
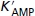 |
AMP concentration for half-maximal activation of GPb | 1.4 × 10−5md | 3.54 × 10−6m | 16.4 | 1.4 × 10−5md | 5.31 × 10−6m | 22.49 |
| nH | Hill coefficient for AMP activation of GPb | 1.75d | 1.88 | – | 1.75d | 1.62 | – |
| βfixed | Intrinsic buffer capacity (non-phosphate, non-bicarbonate) | –e | 0.016 m | 72.44 | –e | 0.0034 m | 35.96 |
| VMCT | Vmax of MCT | –e | 4.7 × 10−3m min−1 | 74.42 | –e | 7.47 × 10−4m min−1 | 95.05 |
a, derived from equivalent ATPase fluxes from experimental measurement of ATPase rate in Fig. 4 using ATPase flux =KATP×[ATP] and converting to the stated units; b, derived from the rate constant for PCr resynthesis from experimental data (0.12 min−1 for EDL and 0.22−1 for SOL) and the approximation: Vmax= (resting PCr) × rate constant; c, Jeneson et al. 1996; d, Crerar et al. 1995; e, no reported data for mouse EDL and SOL.
