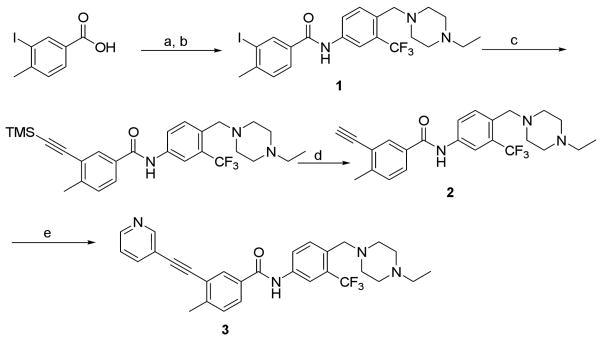
Scheme 1.
Synthetic route of 3.a
a Reagents and conditions: (a) SOCl2, reflux, 1h; (b) 4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)-benzenamine, DIEA, CH2Cl2, 0 °C to RT, 56% over two steps; (c) ethynyltrimethylsilane, Pd(PPh3)4, CuI, DIEA, DMF, RT, 62%; (d) TBAF, THF, RT, 72%; (e) 3-iodopyridine, Pd(PPh3)4, CuI, DIEA, DMF, 50 °C, 72%.