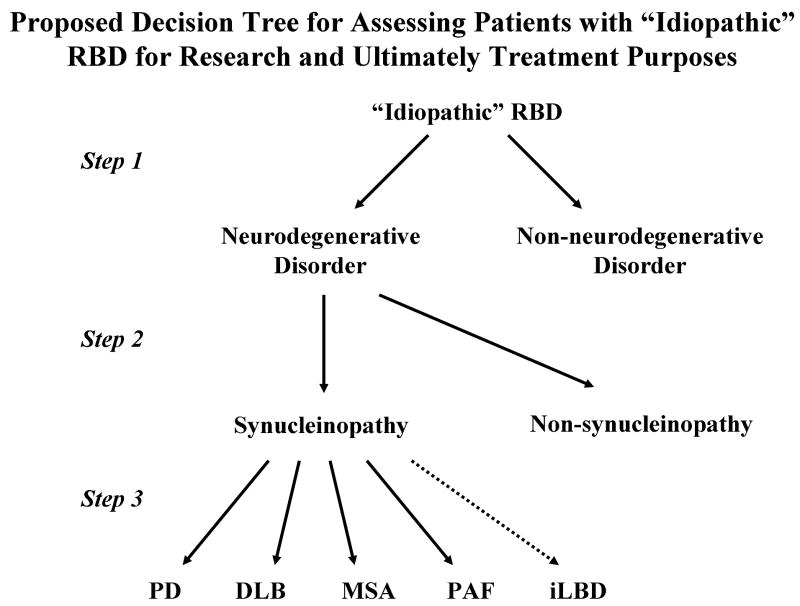
Figure 10.
Proposed decision tree for assessing patients with “idiopathic” RBD for research and ultimately treatment purposes. Presuming that one of the ultimate goals of identifying patients with apparently “idiopathic” RBD is to commence one or more therapies which could delay the onset of cognitive impairment and/or parkinsonism, slow down the course, or halt progression of an underlying neurodegenerative disorder altogether, one would first seek to determine in individual patients who is manifesting features of an early neurodegenerative disorder and who is not (step 1). Perhaps many of the clinical features and ancillary test findings covered in this review, and hopefully many others yet to be developed, will function as accurate biomarkers. Next, if the biomarkers are sufficiently specific to pinpoint the underlying proteinopathy (ie, synucleinopathy vs non-synucleinopathy), then such biomarkers will function in steps 1 and 2; if not, new biomarkers with adequate sensitivity and specificity for proteinopathies and particularly synucleinopathies will need to be developed. Finally, it remains to be seen if agents that ultimately affect synucleinopathy pathophysiology will be safe and efficacious in all of the synucleinopathies regardless of whether the pathologic process is LBD or MSA, or the phenotype is PD-predominant, DLB-predominant, MSA-predominant, or PAF-predominant. Incidental LBD (iLBD) will be impossible to predict without pathology. The challenge for investigators at present is to study adequate numbers of patients with “idiopathic” RBD with a spectrum of clinical tests and potential biomarkers longitudinally to assess the natural history and prepare for future clinical trials.
Abbreviations: DLB=dementia with Lewy bodies, MSA=multiple system atrophy PAF=pure autonomic failure, PD=Parkinson's disease, RBD=REM sleep behavior disorder