Table 2.
Experimental and calculateda enantioselectivity.b Influence of pyrrolidine conformation on % ee prediction.
| catalyst | experimental % ee (ΔΔG‡) | predicted % ee (ΔΔH‡, ΔΔG‡) | ||
|---|---|---|---|---|
| 8TS | 4TS (up) | 4TS (down) | ||
| 40 (0.5)c |
68 (1.0) 74 (1.1) |
|||
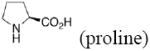 |
72 (1.1) |
69 (1.0) 81 (1.3) |
65 (0.9) 76 (1.1) |
79 (1.3) 92 (1.9) |
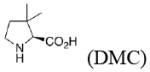 |
unknown |
75 (1.2) 80 (1.3) |
53 (0.7) 63 (0.9) |
82 (1.4) 86 (1.5) |
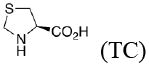 |
73 (1.1) |
82 (1.4) 93 (1.9) |
77 (1.2) 87 (1.6) |
86 (1.6) 95 (2.2) |
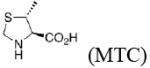 |
unknown |
86 (1.5) 95 (2.1) |
76 (1.2) 82 (1.4) |
87 (1.6) 95 (2.2) |
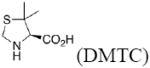 |
86 (1.5) |
87 (1.6) 91 (2.8) |
67 (1.0) 78 (1.2) |
87 (1.6) 92 (1.9) |
B3LYP 6-31G(d,p).
Energy difference in parentheses. Enthalpies in bold; free energies in italics.
Aldehyde is paranitrobenzaldehyde.
