Abstract
Recombinant adenoviruses are employed widely for vascular gene transfer. Vascular smooth muscle cells (SMCs) are a relatively poor target for transgene expression after adenovirus-mediated gene delivery, however, even when expression is regulated by powerful, constitutive viral promoters. The major immediate-early murine cytomegalovirus enhancer/promoter (MIEmCMV) elicits substantially greater transgene expression than the human cytomegalovirus promoter (MIEhCMV) in all cell types in which they have been compared. The Woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) increases transgene expression in numerous cell lines, and fragments of the smooth muscle myosin heavy chain (SMMHC) promoter increase expression within SMC from heterologous promoters. We therefore, compared the expression of β-galactosidase after adenovirus-mediated gene transfer of lacZ under the transcriptional regulation of a variety of combinations of the promoters and enhancers described, in vitro and in porcine coronary arteries. We demonstrate that inclusion of WPRE and a fragment of the rabbit SMMHC promoter along with MIEmCMV increases β-galactosidase expression 90-fold in SMC in vitro and ≈40-fold in coronary arteries, compared with vectors in which expression is regulated by MIEhCMV alone. Expression cassette modification represents a simple method of improving adenovirus-mediated vascular gene transfer efficiency and has important implications for the development of efficient cardiovascular gene therapy strategies.
Keywords: smooth muscle cells, recombinant adenoviral vectors, gene transfer
Introduction
Gene therapy offers great promise as a therapeutic modality for a range of cardiovascular diseases. Its use is being pursued in areas as diverse as prevention of accelerated atherosclerosis, inhibition of atherosclerotic plaque rupture, suppression of cardiac allograft rejection and angiogenic therapy for myocardial and limb ischaemia. As the principal cell type of the arterial wall, the vascular smooth muscle cell (SMC) is the cellular target in many of these settings. Recombinant adenoviruses (RAd) of human serotypes 2 and 5 are among the most widely used vectors for vascular gene transfer because of their relatively high transduction efficiency,1 wide tropism, ability to infect quiescent cells and ease of production.2 Unfortunately, SMCs are largely refractory to gene transfer mediated by adenoviruses of serotypes 2 and 5. This is due to poor viral entry and inefficient transcription of the transgene itself. Reduced viral entry is explained largely by the limited expression of the coxsackie adenovirus receptor (CAR) on the surface of SMC, which results in transduction levels that are markedly lower than those achieved in epithelial cells infected at the same dose.3
Inefficiency of transgene expression in SMC is exemplified by the major intermediate-early human cytomegalovirus enhancer/promoter (MIEhCMV). Despite being among the most potent promoters of transgene expression, the MIEhCMV remains largely inactive in cultured human SMC. After adenovirus-mediated gene transfer at a multiplicity of infection (MOI) of 10, only a small proportion (≈5%) of infected SMC expresses detectable levels of transgene when transcriptional regulation is governed by the MIEhCMV.4 Other viral promoters such as the Rous Sarcoma Virus long terminal repeat promoter or Simian Virus 40 early promoter typically give rise to less transgene expression in SMC than MIEhCMV.5 Transcriptional targeting employing SMC-specific promoters has successfully conferred cell-type specificity,6 but the magnitude of transgene expression achieved using the SMC-specific SM22α promoter was less than that achieved using MIEhCMV.7,8
The level of transgene expression induced by the major intermediate-early enhancer/promoter from the murine cytomegalovirus (MIEmCMV) in a range of murine, human, canine, rat and monkey cell types in vitro was five- to 22-fold greater than that achieved by MIEhCMV.8 In contrast to MIEhCMV, the 1.4 kb form of the murine promoter is capable of inducing detectable levels of transgene expression after target cell transduction by a single-gene transfer vector.9
The post-transcriptional regulatory element of the Woodchuck Hepatitis B Virus (WPRE) increases levels of nuclear transcripts and transgene expression five- to eight-fold in a variety of cells of non-SMC origin, when inserted in a sense orientation in the 3′-untranslated region.10 This effect is promoter- and transgene-independent.10
A short fragment of the rabbit smooth muscle myosin heavy chain (SMMHC) promoter also enhances transgene expression from heterologous promoters in vascular SMC.11 This 107-bp fragment (rabbit SMMHC enhancer; RE) increased transgene expression from the Simian Virus 40 early promoter and from the herpes simplex virus thymidine kinase promoter by three- to eight-fold and seven- to 11-fold, respectively, in primary cultured SMC. No effect was observed in cell lines of non-SMC origin, in nonvascular SMC or in vascular SMC cell lines not expressing the SMMHC gene. An equivalent enhancer fragment from the mouse SMMHC promoter has also been identified (mouse SMMHC enhancer=ME).12
We have previously demonstrated that MIEmCMV gives rise to significantly greater expression of β-galactosidase in cultured SMC than MIEhCMV.13 In the present study, we have investigated the magnitude of transgene expression achieved in primary cultured SMC and in pig coronary arteries by adenovirus-mediated transfer of lacZ under the regulation of combinations of MIEmCMV, WPRE and either RE or ME, and compared this with the transgene expression achieved by MIEhCMV. We demonstrate that inclusion of MIEmCMV, WPRE and RE within the expression cassette of a first-generation adenovirus vector increases transgene expression in vascular SMC in vitro over 90-fold and in pig coronary arteries approximately 40-fold, compared to a vector in which transcriptional regulation is under the control of MIEhCMV alone.
Results
X-gal staining of SMC in vitro
Five vectors, Ad5-lacZ, RAd36, Ad5-PP-lacZ, Ad5-PMEP-lacZ and Ad5-PREP-lacZ, were studied. All are first-generation recombinant serotype 5 adenoviruses (Ad5) containing the gene for E. coli β-galactosidase, lacZ. The combinations of promoter and enhancers within the expression cassette of each virus are presented in Table 1 and Figure 1. To determine the relative efficiency of each vector at effecting transgene expression in vascular SMC, β-galactosidase expression was assessed by X-gal cytochemistry in primary cultured porcine aortic SMC infected with each vector at a range of MOI.
Table 1.
Promoter and enhancer elements present in each vector
| Adenovirus | CMV promoter | Enhancer(s) | Particle:pfu ratio |
|---|---|---|---|
| Ad5-lacZ | Human | – | 34 |
| RAd36 | Murine | – | 35 |
| Ad5-PP-lacZ | Murine | WPRE | 29 |
| Ad5-PMEP-lacZ | Murine | WPRE; ME | 30 |
| Ad5-PREP-lacZ | Murine | WPRE; RE | 45 |
All vectors express lacZ. Human, major immediate-early human CMV enhancer/promoter (−700 to +50); Murine, major immediate-early murine CMV enhancer/promoter (−1336 to +36 bp); WPRE, Woodchuck hepatitis virus post-transcriptional regulatory element; ME, enhancer fragment of murine smooth muscle myosin heavy chain promoter; RE, enhancer fragment of rabbit smooth muscle myosin heavy chain promoter.
Figure 1.
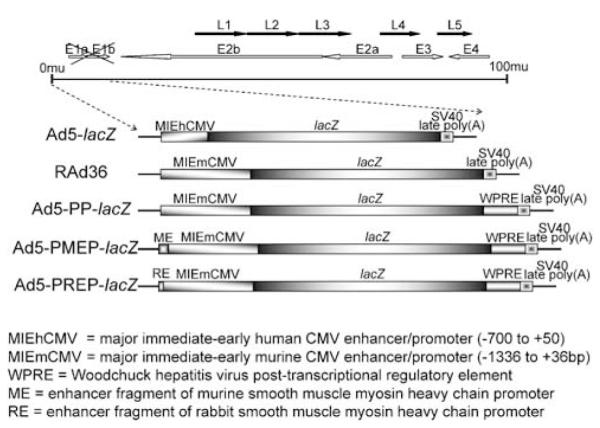
Schematic representation of the transgene expression cassette within each of the vectors studied.
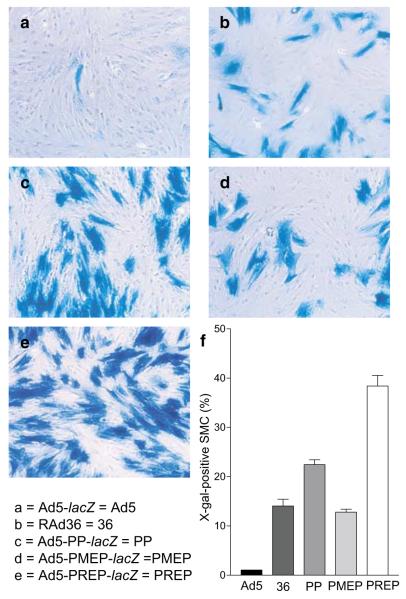
Gene transfer by Ad5-lacZ at MOI=10 resulted in transgene expression in 1.04±0.01% of SMC (Figure 2a and f). RAd36 (Figure 2b and f) gave rise to an approximately 14-fold increase in the number of cells expressing β-galactosidase (14.05±1.35%, P<0.001). Ad5-PP-lacZ gave rise to X-gal staining in 22.43±0.97% of cells (P<0.001 compared to RAd36; Figure 2c and f), although Ad5-PMEP-lacZ did not elicit a significant rise in the proportion of transduced cells by comparison to RAd36 (12.79±0.61%; Figure 2d and f). Ad5-PREP-lacZ induced transgene expression in 38.36±2.13% of SMC infected at MOI=10 (Figure 2e and f). This is 37-fold greater than Ad5-lacZ, approximately three-fold greater than RAd36 and Ad5-PMEP-lacZ and approximately two-fold greater than Ad5-PP-lacZ (all P<0.001) (Figure 2f). At MOI=500, ≈100% of SMC were β-galactosidase positive regardless of vector type.
Figure 2.
Expression of β-galactosidase in SMC after infection with recombinant adenoviral vectors. PASMC infected with (a) Ad5-lacZ, (b) RAd36, (c) Ad5-PP-lacZ, (d) Ad5–PMEP-lacZ, (e) Ad5–PREP-lacZ after cytochemical staining with X-gal. All cells were infected at MOI=10. (f) The proportion of β-galactosidase-positive cells 72 h post-infection is significantly greater after infection with Ad5–PREP-lacZ than with any other vector (all P<0.001).
β-galactosidase activity in cell lysates in vitro
To further assess the efficiency of vector-mediated transgene delivery, β-galactosidase activity in SMC lysates infected at MOI=10 and 500 was measured quantitatively by a chemilucent biological assay. Activity was also assessed in COS-7 cells (a non-SMC cell line) infected at MOI=10.
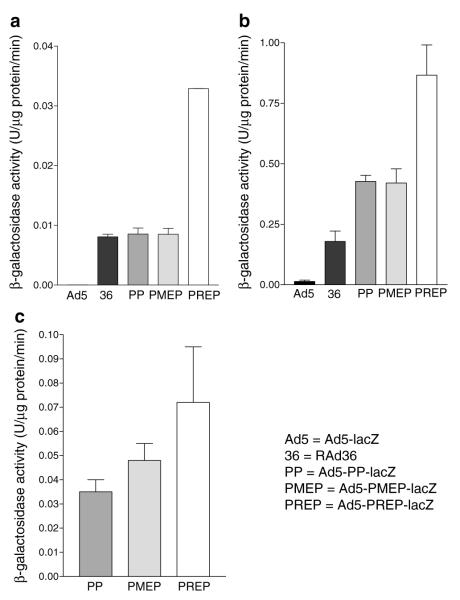
At low MOI (MOI=10) Ad5-lacZ-induced activity was almost negligible (4.6×10−5 U/μg/min; Figure 3a). All vectors containing MIEmCMV induced significantly greater activity than Ad5-lacZ (Figure 3a; all P<0.001). No significant difference was observed between the activities induced by RAd36, Ad5-PP-lacZ and Ad5-PMEP-lacZ. Ad5-PREP-lacZ, however, induced approximately four-fold greater activity than RAd36, Ad5-PP-lacZ and Ad5-PMEP-lacZ and several hundred-fold increased activity compared to Ad5-lacZ (Figure 3a; all P<0.0001).
Figure 3.
β-galactosidase activity in lysates of cultured SMC and COS-7 cells. At MOI 10 (a), all MIEmCMV-based vectors give rise to greater activity than the MIEhCMV-based vector Ad5-lacZ (all P<0.001). Activity is significantly greater in Ad5-PREP-lacZ infected cells than all others (all P<0.0001). At MOI 500 (b), β-galactosidase activity is significantly greater in Ad5-PREP-lacZ-infected cells than all other groups (all P<0.001), almost five-fold greater than that induced by RAd36, and almost 90-fold greater than Ad5-lacZ. In fibroblasts (COS-7) infected at MOI 10 (c), there is no significant difference between the β-galactosidase activity induced by any of the MIEmCMV-based vectors containing WPRE (P=0.25).
At high MOI (MOI=500), all MIEmCMV-based vectors induced significantly greater β-galactosidase activity than Ad5-lacZ (all P<0.001; Figure 3b). β-galactosidase activity in RAd36-infected SMC was 18-fold greater than in Ad5-lacZ-infected cells (Figure 3b; P<0.01). All vectors including the WPRE induced greater activity than RAd36 (all P<0.01; Figure 3b). Greatest transgene expression was observed in SMC infected with Ad5-PREP-lacZ. β-galactosidase activity in Ad5-PREP-lacZ-infected cells was ≈90-fold greater than that in Ad5-lacZ-infected cells, five-fold greater than RAd36-infected cells and two-fold greater than the expression observed in Ad5-PP-lacZ- and Ad5-PMEP-lacZ-infected cells (Figure 3b; all P<0.001).
In contrast, β-galactosidase activity in COS-7 cells did not differ significantly after infection at MOI=10 with Ad5-PP-lacZ, Ad5-PMEP-lacZ or Ad5-PREP-lacZ (Figure 3c).
Expression of β-galactosidase in pig coronary arteries
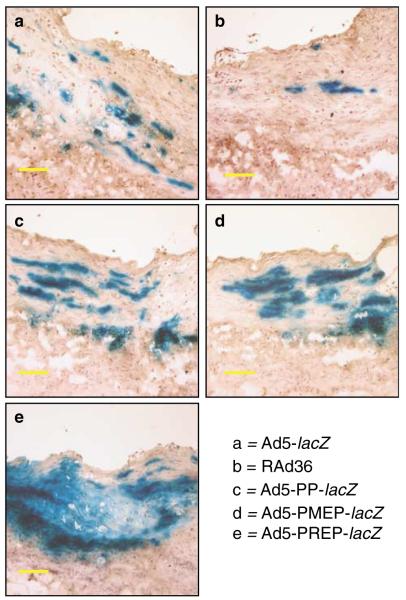
Four coronary arteries were infected with 2×109 iu of each virus in a volume of 300 μl. Histological examination of sections of infected artery revealed transgene expression after Infiltrator™ catheter-mediated delivery of each recombinant vector to be principally within the outer media and adventitia. Immunohistochemical staining revealed β-galactosidase to be co-localized in those areas of vessel wall staining for SM α-actin (Figure 4). The expression of β-galactosidase as quantified by the area of X-gal staining in sections of vessel was significantly different between vector groups (P<0.0001; Figure 5a).
Figure 4.
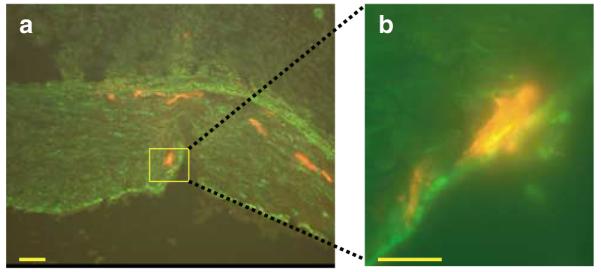
Immunohistochemistry of an Ad5-PREP-lacZ infected vessel. Sections of porcine coronary artery underwent immunofluorescent staining 72 h after infection with Ad5-PREP-lacZ. (a) Vessel costained for SM α-actin (green) and β-galactosidase (red). Vessel histology is demonstrated. Red fluorescence is seen within the media. (b) Vessel at higher power showing colocalization of the cytoskeletal α-actin stain and the cytosolic β-galactosidase stain (bars=50 μm).
Figure 5.
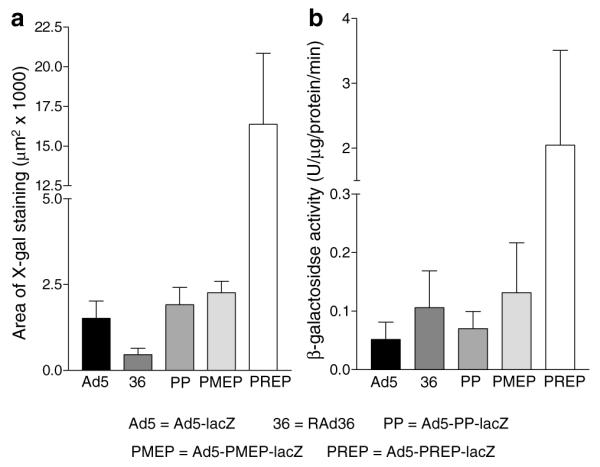
β-Galactosidase expression in infected coronary vessels. (a) The area of X-gal staining 72 h after exposure to each virus. Staining is significantly greater in vessels infected with Ad5–PREP-lacZ than with any other vector (all P<0.001). (b) β-galactosidase activity in lysates of coronary arteries 72 h after Infiltrator-mediated delivery of adenovirus vectors. Ad5-PREP-lacZ-infected vessels revealed ≈40-fold greater β-galactosidase activity than Ad5-lacZ-infected vessels (P<0.05) and ≈20-fold greater activity than RAd36-, Ad5-PP-lacZ- and Ad5-PMEP-lacZ-infected vessels (all P<0.01).
The greatest area of X-gal staining was observed in Ad5-PREP-lacZ-infected vessels (Figure 6e; 16381±4483 μm2), being 10-fold greater (P<0.001) than the area of staining in Ad5-lacZ-infected vessels (Figure 6a; 1519±503 μm2). Areas of X-gal staining were not significantly greater in RAd36-, Ad5-PP-lacZ- or Ad5-PMEP-lacZ-infected arteries than in Ad5-lacZ-infected vessels (Figures 5a and 6a–d).
Figure 6.
β-Galactosidase expression in infected coronary arteries. Sections of coronary artery stained with X-gal 72 h after Infiltrator-mediated infection with each of the vectors used. The greatest area of β-galactosidase staining is observed in the Ad5-PREP-lacZ-treated vessels (e) (bars=50 μm).
The biological activity of β-galactosidase was distributed in a similar fashion to the area of X-gal staining in each vessel (Figure 5b). Activity was 0.05±0.02 U/μg/min in homogenates of Ad5-lacZ-infected vessels. RAd36 infection induced approximately twice the β-galactosidase activity observed in the Ad5-lacZ group. This was not statistically significant. β-Galactosidase activity was not increased by Ad5-PP-lacZ or Ad5-PMEP-lacZ compared to RAd36. Levels of β–galactosidase activity in Ad5-PREP-lacZ-infected vessels were significantly greater than in all other groups (P=0.007; Figure 5b). Ad5–PREP–lacZ induced 40-fold greater β-galactosidase activity than Ad5-lacZ (P<0.05), ≈20-fold greater than RAd36 (P<0.01) and 30- and 15-fold greater activity than Ad5-PP-lacZ and Ad5-PMEP-lacZ, respectively (both P<0.01).
Discussion
We have investigated the ability of MIEmCMV, WPRE, ME and RE to increase transgene expression in SMC. We have demonstrated that a recombinant Ad5 in which transgene expression is under the regulation of MIEmCMV, WPRE and RE increased the level of transgene expression in primary cultures of porcine SMC by 90-fold, and in pig coronary arteries by ≈40-fold compared to MIEhCMV alone. At MOI=500 in vitro, when all vectors gave rise to transgene expression in virtually all SMC, Ad5-PREP-lacZ-mediated gene transfer induced ≈90-fold greater transgene expression than Ad5-lacZ, demonstrating that transgene expression in each cell is significantly increased by the PREP expression cassette.
These modifications to the expression cassette do not alter vector tropism. All of the vectors studied may infect vessel wall cells other than SMC. This raises the possibility that increased transgene expression from Ad5-PREP-lacZ in vivo may be because of increased expression in cells other than SMC. However, transgene expression in fibroblasts did not differ significantly following infection with Ad5-PREP-lacZ, Ad5-PP-lacZ or Ad5-PMEP-lacZ in vitro. This suggests that differences observed following in vivo infection are more likely to be because of variations in expression within SMC, in which significantly greater β-galactosidase activity was induced in vitro by Ad5-PREP-lacZ than by Ad5-PP-lacZ or Ad5-PMEP-lacZ. Furthermore, the immunohistochemical analysis illustrated in Figure 4 demonstrates that β-galactosidase expression colocalised with cells staining positively for SM α-actin. Within the media, such cells are almost certainly SMC. Adventitial fibroblasts are implicated in neointimal hyperplasia however,14 and therapeutic expression of transgene by these cells would not be undesirable within the coronary vasculature postinjury.
MIEhCMV has been widely used in vascular gene transfer on the basis that it is one of the most powerful promoters of transgene expression available. However, pharmacological enhancement increased the proportion of SMC expressing transgene following gene transfer at MOI=10 from 5 to 55%, suggesting, that in the absence of enhancer stimulation, >90% of copies of MIEhCMV entering SMC fail to express transgene.4 Unfortunately, these methods of enhancement are not well suited to in vivo application. Furthermore, the ideal vector for vascular gene therapy should allow maximal transgene expression after a single dose without further physical or pharmacological manipulations. We have demonstrated that MIEmCMV induces significantly greater transgene expression in SMC than MIEhCMV without pharmacological enhancement.
The rabbit smooth muscle-specific enhancer was first identified as regulating tissue-restricted expression of the SMMHC gene.11 Addition of RE to a vector in which transgene expression is driven by MIEmCMV and WPRE improves the expression four- to five- fold in SMC in vitro. At MOI=500 Ad5-PREP-lacZ induced approximately two-fold greater transgene expression than Ad5–PP–lacZ, demonstrating a significant increase in transgene expression in each cell as a consequence of inclusion of RE. Enhancement of transgene expression was not observed in the presence of RE in COS-7 cells, a fibroblast cell line that expresses CAR. In contrast, inclusion of ME failed to increase transgene expression in cultured SMC or in vivo. This enhancer fragment is larger than RE (211 versus 107 bp) and, while it contains most of the sequence included in RE, it may also contain elements that have an inhibitory effect on transgene expression.12 The rat SMMHC promoter contains a negative regulatory GC-rich element (CCCGCCC), mutation of which increases expression of CAT in SMC by 100%.15 The same sequence is present in the ME sequence.
The SM22α promoter has previously been studied as a means of restriction of transgene expression to SMC,6 however, this promoter gives rise to transgene expression ≈103-fold lower than the MIEhCMV promoter in vivo.16 Ribault et al confirmed the poor performance of the SM22α promoter alone compared to MIEhCMV, but observed that a combination of RE and the SM22α promoter improved transgene expression in vivo to the level achieved by MIEhCMV.7 In vivo interferon-γ expression driven by the SMMHC enhancer/SM22α chimeric promoter reduced neointimal hyperplasia in injured rat carotid arteries to the same extent as that driven by the MIEhCMV promoter, but no more marked effect was observed.7 As we have demonstrated, however, the levels of transgene expression induced by MIEhCMV in SMC are substantially less than the maximum achievable.
Inclusion of the WPRE in addition to MIEmCMV increased levels of X-gal staining or β-galactosidase activity by two-fold in SMC compared to MIEmCMV alone at MOI=500, but no improvement was observed at MOI=10 or in vivo. As we did not observe any reduction in transgene expression from Ad5-PP-lacZ- compared to RAd36, however, inclusion of WPRE is clearly not detrimental to the levels achieved.
Previous attempts to increase transgene expression after adenovirus-mediated gene transfer into SMC have focused on modification of vector tropism to increase the efficiency of transgene entry to the cell. Mutations of the fibre protein to include an RGD-motif17 (allowing binding to αv integrins), a poly-lysine sequence17 (to enhance binding to heparan-sulphate containing receptors), the Ad3 fibre protein18 and the Ad16 fibre protein19 have all increased transgene expression in SMC in vitro. The latter two vectors, however, were associated with reduced transgene expression in pig and/or rat SMC and are clearly of limited use in animal studies where rat carotid and pig coronary and ilio-femoral arteries are commonly used targets for gene transfer. The expression cassette in Ad5-PREP-lacZ gives rise to unequivocally increased transgene expression in pig SMC and in pig coronary arteries. Furthermore, this expression cassette is compatible with any of the modified vectors described, offering the potential for generation of vectors that could increase transgene expression in SMC by several hundred-fold in vivo.
The use of MIEmCMV to promote transgene expression within the vasculature represents a major advance over the use of previously available heterologous promoters. Inclusion of WPRE and a short fragment of the rabbit SMMHC promoter within the expression cassette of a recombinant adenovirus driven by this strong viral promoter substantially improves transgene expression both in vitro and in porcine coronary arteries. By allowing considerable increases in transgene expression from a given dose of vector, these results have very important implications for the successful application of gene therapy within the vasculature.
Materials and methods
Generation of recombinant adenoviruses
Five vectors, Ad5-lacZ, RAd36, Ad5-PP-lacZ, Ad5-PMEP-lacZ and Ad5-PREP-lacZ, were studied. The combinations of promoter and enhancers within the expression cassette of each virus are presented in Table 1 and Figure 1. Generation of Ad5-lacZ and RAd36 has been described previously.9,20,21 Ad5-PP-lacZ, Ad5-PMEP-lacZ and Ad5-PREP-lacZ were generated by homologous recombination in 293 cells.22 Stocks were purified by CsCl gradient centrifugation and titered by serial dilution end point in HEK 293 cells.22 Each preparation was titered in triplicate by three independent investigators and particle:pfu ratios calculated.22 Expression of β-galactosidase in the end-point dilution wells of each vector was confirmed by X-gal staining.
Cell culture and infection
Primary cultures of porcine aortic SMC were established as described previously.20 SMC were grown to 90% confluence in six-well plates and rendered quiescent by incubation in low-serum medium (2% FCS) for 48 h prior to infection. SMCs were infected with each virus at MOIs of 10 and 500.
COS-7 cells were grown in six- well plates until 90% confluent. They were then exposed to low-serum medium for 48 h after which they were infected with each virus at an MOI of 10.
Assessment of β-galactosidase activity
At 48 h postinfection, the proportions of porcine SMC expressing β-galactosidase after infection at MOI=10 with each virus were assessed by X-gal cytochemistry.23 Quantitative β-galactosidase activities in lysates of SMC infected at MOI=10 and 500, and COS-7 cells infected at MOI=10 were assessed by a chemilucent biological assay. The protein content in each cell lysate was assayed and β-galactosidase activity expressed as units of β-galactosidase/μg protein/minute (U/μg/min).23
Intracoronary adenovirus delivery
In all, 20 large white pigs (19–25 kg) underwent intracoronary injection of virus. Procedures conformed to the UK Animals (Scientific Procedures) Act 1986 and were authorized by the Home Office. Animals received 150 mg of aspirin 24 h before, and 48 h post-PTCA. Before each procedure, vessels were randomized to receive 2 × 109 iu of Ad5-lacZ RAd36, Ad5-PP-lacZ, Ad5-PMEP-lacZ or Ad5-PREP-lacZ in 300 μl volume. Anaesthesia was induced by isoflurane inhalation. An endotracheal tube was inserted and anaesthesia maintained using 2% isoflurane. The left carotid artery was exposed, an 8F guide catheter inserted and 2500 iu of heparin injected. Glyceryl trinitrate (200 μg) was injected into the left coronary artery and angiography performed using a digital image intensifier. Segments of the left anterior descending artery 2.0–2.5 mm in diameter, over 15 mm long and with no significant side branches were infected using a 3.0 mm Infiltrator™ catheter (Interventional Technologies). Vector was injected over a typical period of ≈30 s. A postdelivery angiogram was performed. The carotid artery was ligated, the neck incision closed and the animals allowed to recover. Animals were euthanized 72 h after PTCA. Blocks from the centre of each infected segment were embedded in OCT and snapfrozen in liquid nitrogen for X-gal staining. The remaining lengths of each infected vessel segment were frozen in liquid nitrogen prior to β-galactosidase assay.
Transgene expression in vivo
X-gal cytochemistry23 was performed on 7 μm-thick cryostat sections. The area of X-gal staining in sections from each vessel was measured using a Leica Quantimet 600s digital analysis system by an operator blinded to the vector with which each vessel had been infected. Frozen vessel segments were homogenized in liquid nitrogen and resuspended in lysis solution (25 mm Tris-HCl (pH 7.8), 15% glycerol v/v, 7.5 ml; 10 mm MgCl2, 1% Triton X-100 v/v, 1 mm EDTA (pH 8). β-galactosidase activity was assessed as described above in vessel homogenates.
Immunohistochemistry was performed in 7 μm-thick cryostat sections. Sections were exposed to primary anti-SM α-actin and anti-β-galactosidase antibodies. Secondary labelling was performed using FITC- and Texas Red-conjugated antibodies respectively.
Statistical analysis
Multiple groups were analysed by one-way ANOVA. Comparisons were made between groups by Newman–Keuls post hoc test. Differences were considered statistically significant at values of P<0.05.
Acknowledgements
CEA is a British Heart Foundation Junior Research Fellow (FS/2000072).
References
- 1.Laitinen M, et al. Gene transfer into the carotid artery using an adventitial collar: comparison of the effectiveness of the plasmid–liposome complexes, retroviruses, pseudotyped retroviruses and adenoviruses. Hum Gene Ther. 1997;8:1645–1650. doi: 10.1089/hum.1997.8.14-1645. [DOI] [PubMed] [Google Scholar]
- 2.Feldman LJ, Steg G. Optimal techniques for arterial gene transfer. Cardiovasc Res. 1997;35:391–404. doi: 10.1016/s0008-6363(97)00148-x. [DOI] [PubMed] [Google Scholar]
- 3.Wickham TJ, et al. Targeted adenovirus gene transfer to endothelial and smooth muscle cells by using bispecific antibodies. J Virol. 1996;70:6831–6838. doi: 10.1128/jvi.70.10.6831-6838.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clesham GJ, Browne H, Efstathiou S, Wiessberg PL. Enhancer stimulation unmasks latent gene transfer after adenovirus-mediated gene delivery into human vascular smooth muscle cells. Circ Res. 1996;79:1188–1195. doi: 10.1161/01.res.79.6.1188. [DOI] [PubMed] [Google Scholar]
- 5.De Young MB, et al. Optimizing vascular gene transfer of plasminogen activator inhibitor 1. Hum Gene Ther. 1999;10:1469–1478. doi: 10.1089/10430349950017806. [DOI] [PubMed] [Google Scholar]
- 6.Kim S, et al. Transcriptional targeting of replication-defective adenovirus transgene expression to smooth muscle cells in vivo. J Clin Invest. 1997;100:1006–1014. doi: 10.1172/JCI119611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribault S, et al. Chimeric smooth muscle-specific enhancers/promoters – valuable tools for adenovirus-mediated cardiovascular gene therapy. Circ Res. 2001;88:468–475. doi: 10.1161/01.res.88.5.468. [DOI] [PubMed] [Google Scholar]
- 8.Addison CL, Hitt M, Kunsken D, Graham FL. Comparison of the human versus murine cytomegalovirus immediate early gene promoters for transgene expression by adenoviral vectors. J Gen Virol. 1997;78:1653–1661. doi: 10.1099/0022-1317-78-7-1653. [DOI] [PubMed] [Google Scholar]
- 9.Gerdes CA, Castro MG, Lowenstein PR. Strong promoters are the key to highly efficient, noninflammatory and noncytotoxic adenoviral-mediated transgene delivery into the brain in vivo. Mol Ther. 2000;2:330–338. doi: 10.1006/mthe.2000.0140. [DOI] [PubMed] [Google Scholar]
- 10.Zufferey R, Donello J, Trono D, Hope T. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J Virol. 1999;73:2886–2892. doi: 10.1128/jvi.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kallmeier RC, Somasundaram C, Babij P. A novel smooth muscle-specific enhancer regulates transcription of the smooth muscle myosin heavy chain gene in vascular smooth muscle cells. J Biol Chem. 1995;270:30949–30957. doi: 10.1074/jbc.270.52.30949. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe M, et al. Structure and characterization of the 5′-flanking region of the mouse smooth muscle myosin heavy chain (SM1/2) gene. Circ Res. 1996;78:978–989. doi: 10.1161/01.res.78.6.978. [DOI] [PubMed] [Google Scholar]
- 13.Kingston PA, et al. The murine cytomegalovirus immediate-early enhancer/promoter is a more potent regulator of transgene expression than the human promoter in vascular smooth muscle cells in vitro. J Am Coll Cardiol. 2001;37(Suppl A):266A. (Abstract) [Google Scholar]
- 14.Li G, et al. Direct in vivo evidence demonstrating neointimal migration of adventitial fibroblasts after balloon injury of rat carotid arteries. Circulation. 2000;101:1362–1365. doi: 10.1161/01.cir.101.12.1362. [DOI] [PubMed] [Google Scholar]
- 15.Madsen CS, et al. Expression of the smooth muscle myosin heavy chain gene is regulated by a negative-acting GC-rich element located between two positive-acting serum response factor-binding elements. J Biol Chem. 1997;272:6332–6340. doi: 10.1074/jbc.272.10.6332. [DOI] [PubMed] [Google Scholar]
- 16.Akyurek LM, et al. SM22α promoter targets gene expression to vascular smooth muscle cells in vitro and in vivo. Mol Med. 2000;6:983–991. [PMC free article] [PubMed] [Google Scholar]
- 17.Wickham TJ, et al. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fibre proteins. J Virol. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su E, et al. A genetically modified adenoviral vector that exhibits enhanced gene transfer of human smooth muscle cells. J Vasc Res. 2001;38:471–478. doi: 10.1159/000051080. [DOI] [PubMed] [Google Scholar]
- 19.Havenga M, et al. Improved adenoviral vectors for infection into cardiovascular tissues. J Virol. 2001;75:3335–3342. doi: 10.1128/JVI.75.7.3335-3342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kingston PA, et al. Adenovirus-mediated transfer of a secreted transforming growth factor-beta type II receptor inhibits luminal loss and constrictive remodelling after coronary angioplasty and enhances adventitial collagen deposition. Circulation. 2001;104:2595–2601. doi: 10.1161/hc4601.099405. [DOI] [PubMed] [Google Scholar]
- 21.Southgate TD, et al. Transcriptional targeting to the anterior pituitary lactotrophic cells using recombinant adenovirus vectors in vitro and in vivo in normal and estrogen/sulphiride-induced hyperplastic anterior pituitaries. Endocrinology. 2000;141:3493–3505. doi: 10.1210/endo.141.9.7639. [DOI] [PubMed] [Google Scholar]
- 22.Southgate TD, Kingston PA, Castro MG. Gene transfer into neural cells in vitro using adenoviral vectors. In: Crawley JN, et al., editors. Current Protocols in Neuroscience. Suppl 13. John Wiley and Sons, Inc.; New York, NY: 2000. pp. 4.23.1–4.23.40. [DOI] [PubMed] [Google Scholar]
- 23.MacGregor GR. Use of E.coli lacZ (β-galactosidase) as a reporter gene. In: Murray EJ, editor. Methods in Molecular Biology. Vol 7: Gene Transfer and Expression Protocols. The Humana Press Inc.; Clifton, NJ: 1991. pp. 217–235. [DOI] [PubMed] [Google Scholar]