Figure 1.
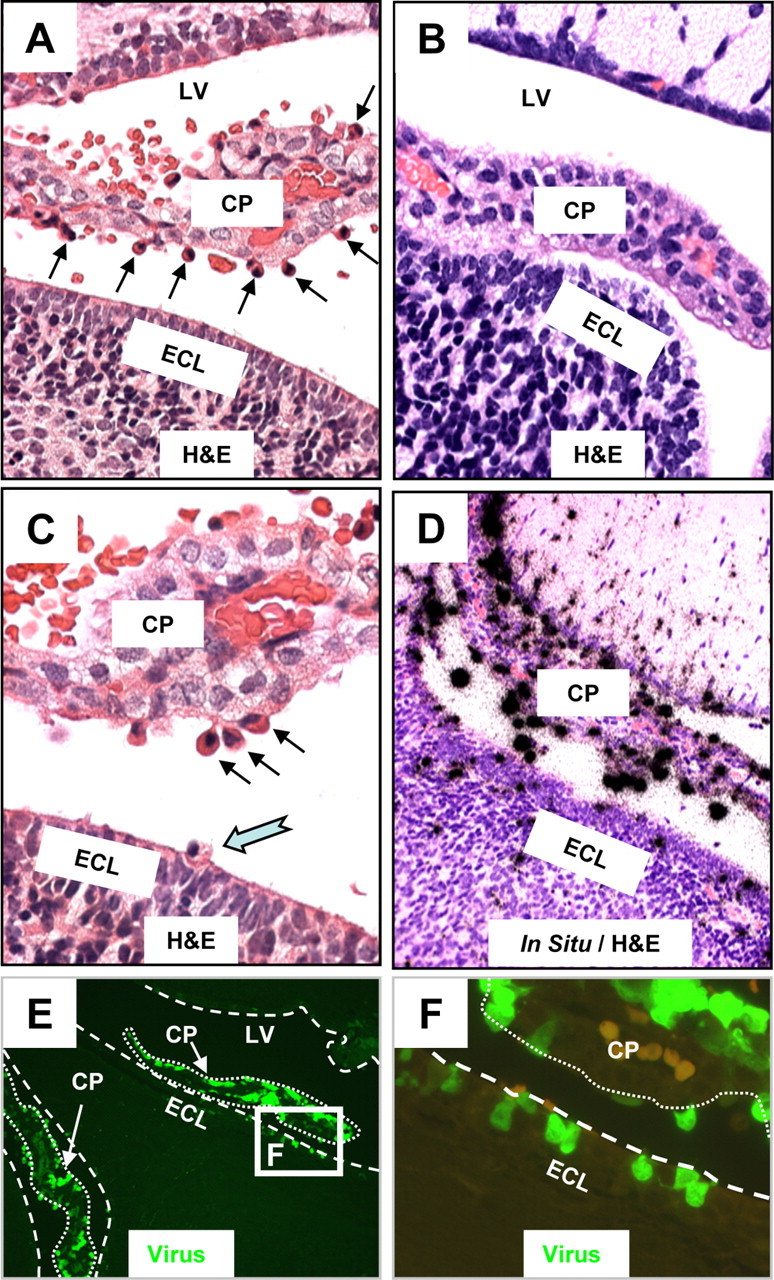
Myeloid cells migrating into the CNS are highly susceptible to CVB3 infection. The brains of mock and eGFP-CVB3-infected neonatal mice (intracranially) were harvested 24 h PI. Brain sections were cut sagittally, deparaffinized, observed by fluorescence microscopy for viral protein expression (eGFP), and stained by H&E (see Materials and Methods). The location of viral RNA was evaluated by in situ hybridization using a 5′-UTR probe specific for CVB3. A, CVB3 infection induced the recruitment of cells having a myeloid-like morphology (black arrows) which migrated from vascular stromal cores through the choroid plexus columnar epithelium. A number of these cells were found within the CSF of the ventricles. B, In contrast to infected mice, the choroid plexus of mock-infected mice revealed no apparent influx of myeloid-like cells near the choroid plexus epithelial cell layer. C, Higher magnification of the infected brain revealed the presence of myeloid-like cells adjacent to the epithelial cell layer of the choroid plexus (black arrows), many of which appeared to migrate through the ECL and into the parenchyma of the brain (cyan notched arrow). D, E, In situ hybridization (D) and fluorescence microscopy (E) demonstrated that these myeloid-like cells found adjacent to the choroid plexus supported robust viral replication (D, black dots) and expressed high levels of viral protein (E, green signal). F, Higher magnification of the white box in E showed multiple infected cells at different stages of diapedesis passing through the lumen of the choroid plexus (outlined with dotted white lines), along with the stepwise migration of these myeloid-like cells across the ECL (outlined with dashed white line). A few autofluorescing red blood cells (light orange) can be seen in the choroid plexus. A, B, D, 40× objective; C, 63× objective; E, 20× objective; F, 100.8× magnification. LV, lateral ventricle.
