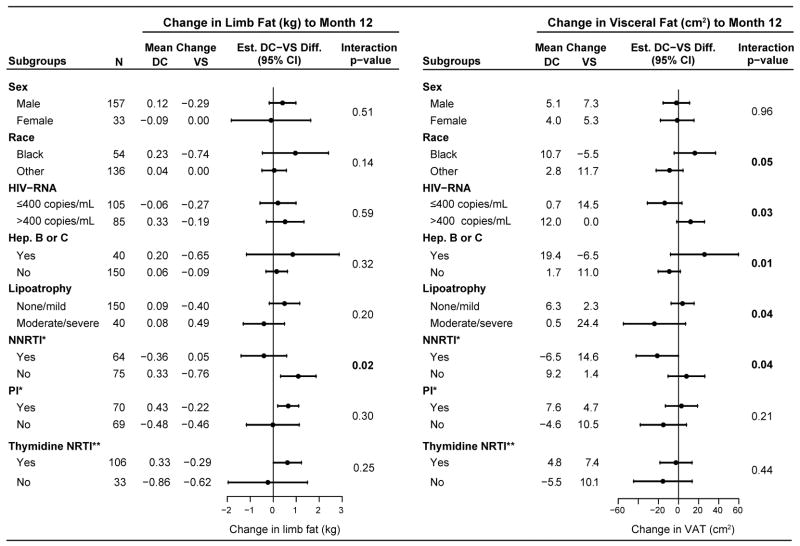
Figure 2.
DC-VS group differences in change in limb fat and VAT from baseline to Month 12 within subgroups of participants, and tests for homogeneity of the treatment difference by subgroup factor.
* NNRTI or PI use, respectively, among those who were receiving ART at baseline.
** Among those who were receiving ART at baseline. In the DC and VS groups, 60 and 46 participants, respectively, used thymidine NRTIs at baseline. In the VS group, 35 (76% of 46) continued using thymidine NRTIs at year 1, and one more had switched to a thymidine NRTI. In the DC group, 24 participants had re-initiated ART by year 1 and 16 were taking thymidine NRTIs.
There was no evidence for differential treatment effect (interaction p-value > 0.05) by the following baseline factors: age, CD4+ count, nadir CD4+ count, prior AIDS, body mass index, smoking, use of ART, or duration of ART use.