Figure 1.
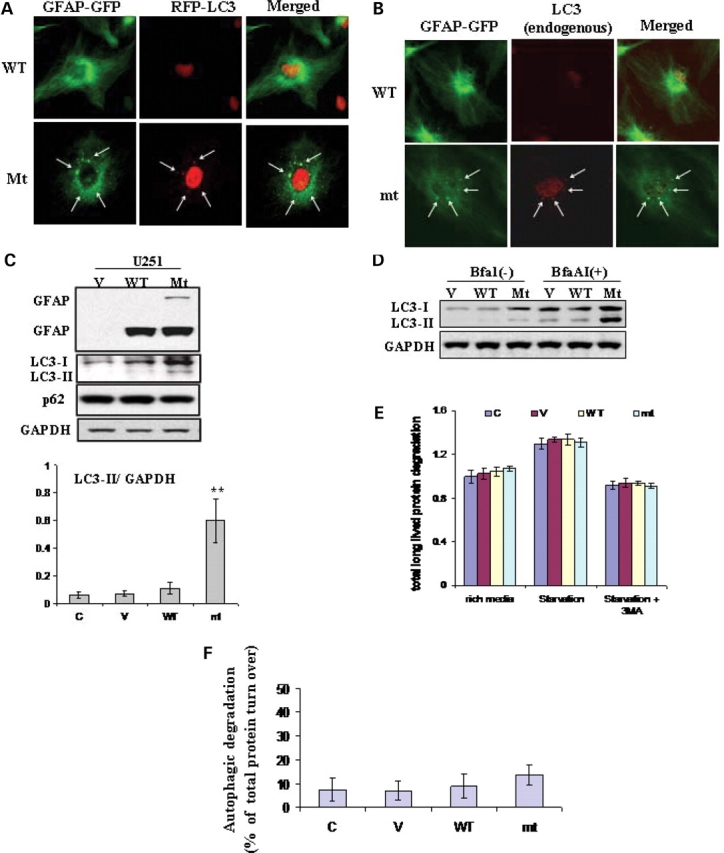
Evidence for autophagy in U251 astrocytoma cells stably expressing R239C AxD mutant GFAP. (A) Co-localization of RFP-LC3 with cytoplasmic GFAP aggregates, indicated by arrows. U251 cells stably expressing EGFP-C1 vector, wt or mt GFAPs were transiently transfected with RFP-LC3. Forty-eight hours after transfection, cells were treated with cytoskeletal buffer and fixed with 4% PFA and then examined for GFAP (green) and LC3 (red) fluorescence. (B) Overlap of endogenous LC3 and GFAP aggregates in GFAP expressing U251 cells. U251 cells were treated with cytoskeletal buffer and fixed with 4% PFA, then were immunostained for LC3. Merged images on right show LC3 in red, GFAP-GFP in green and the overlap in yellow, aggregates were indicated by arrows. (C) Immunoblotting analysis of LC3 in U251 cells stably expressing GFAPs (upper panel). Each gel labeled with V, vector-control; WT, WT GFAP; Mt, mt GFAP. Lower panel: quantitative immunoblot analysis of LC3 activation, represented by LC3-II to GAPDH ratio, under normal growth conditions in U251 astrocytoma cells stably transfected with wild-type and mutant GFAP. ** P < 0.001. (D) Increased autophagic flux in mt GFAP expressing U251cells, represented by the differences between LC3-II level between BfaA1 treated and non-treated cells. (E) Long-lived bulk protein degradation. Compared to U251 cells under normal growth conditions, no significant differences were observed in cells overexpressing wt and mt GFAPs, and cells upon amino acid- and serum-deprivation in the presence or absence of 3-MA macroautophagy inhibitor. n = 6 wells, mean ± SEM two-tailed ANOVA. (F) The proportion of total protein turnover due to autophagy in different cell lines.
