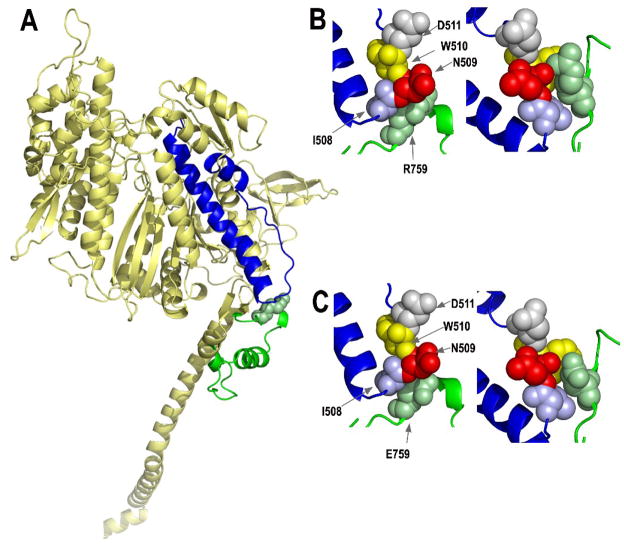
Fig. 1.
Locations of the converter and relay domains of myosin, and the effects of mutating the R759 converter residue. (A) Mapping of the amino acids of the Drosophila indirect flight muscle isoform (IFI) of myosin onto the scallop crystal structure in the pre-power stroke state (1qvi). The relay domain encoded by alternative exon 9a is highlighted in blue, whereas the central portion of the converter domain (residues 724–764) encoded by alternative exon 11e is shown in green. Converter domain residue R759 is shown as a space-filling model. The homology model was produced by fitting the Drosophila indirect flight muscle myosin S-1 amino acid sequence to the coordinates of scallop myosin S1 using the automated mode of the Swiss-Model homology modeling server (http://swissmodel.expasy.org/). PyMOL (http://www.pymol.org/, DeLano Scientific, Palo Alto, CA, USA) was used to visualize the output. (B) Interaction of converter domain residue R759 (green) with amino acid residues of the relay domain [I508 (blue), N509 (red), W510 (yellow), D511 (gray)] in the pre-power stroke state (left) and the post-power stroke state (1kk8; right). Space-filling models suggest the hydrophobic region of R759 near the peptide backbone interacts with I508 in the pre-power stroke state, while the polar terminal portion interacts with polar N509. In the post-power stroke state, interactions with I508 and N509 are retained, plus the changed orientation of the relay loop results in formation of a salt bridge between R759 and D511. See Bloemink et al.30 for further modeling and discussion. (C) Interaction of mutated converter domain residue E759 with amino acid residues of the relay domain in the pre-power stroke state (left) and the post-power stroke state (right). These models were produced as described above, except that E759 replaced R759 prior to modeling. The negatively charged region of E759 is located near hydrophobic I508, eliminating the hydrophobic interaction found in wild-type myosin. The mutation also reduces interaction of residue 759 with polar N509, particularly in the pre-power stroke state (left). Disruption of the relay loop/converter domain interface is exacerbated at the post-power stroke state (right), since E759 is unable to form a salt bridge with negatively charged D511. All residue numbers correspond to those of chicken skeletal muscle myosin.2