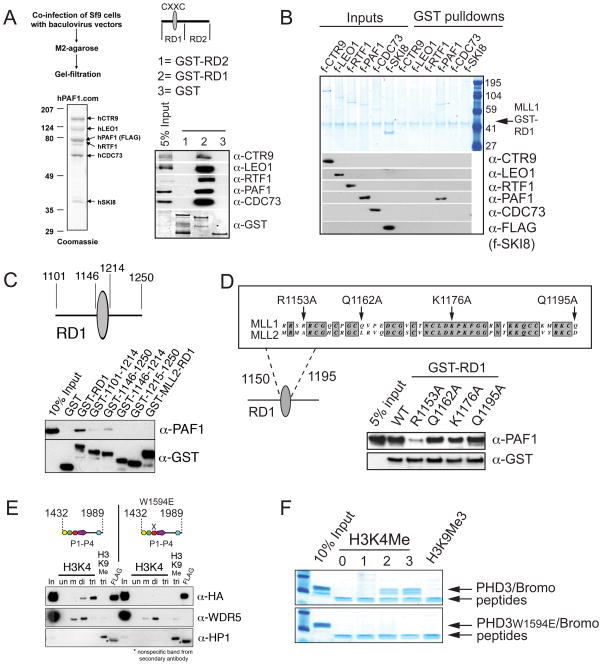
Figure 4. The MLL1 CXXC/RD1 domain interacts directly with PAF1 and PHD finger 3 interacts directly with H3K4Me2/3.
(A) PAF1C interacts directly with the CXXC containing RD1 fragment of MLL1. The MLL1 CXXC region was divided into two fragments (RD1 and RD2) and expressed as recombinant GST proteins. Purified GST (1), GST-RD1 (2) and GST-RD2 (3) were used in GST pulldown experiments with a recombinant purified PAF1C. Westerns were probed with the antibodies indicated. (B) The PAF1 component of PAF1C interacts directly with MLL RD1. GST-RD1 was used in a GST pulldown experiment with individual FLAG (f-) tagged components of human PAF1C as shown. The upper panel is a gel stained with colloidal blue and the lower panels are western blots of the same pulldowns using the antibodies indicated. (C) Only full length MLL1-RD1 interacts efficiently with PAF1, while MLL2-RD1 does not. A series of deletions of the MLL1 RD1 region were made and subjected to GST pulldowns with purified f-PAF1. Westerns were probed with the antibodies indicated. (D) Top: conservation with MLL2 and the positions of point mutations made in the MLL1 CXXC domain are shown. Bottom: The R1153A point mutation disrupts the direct interaction between purified GST-RD1 and purified f-PAF1 protein. GST pulldowns were done as in (A). (E) PHD fingers 1 to 4 with and without a point mutation in PHD3 (W1594E) were FLAG and HA double tagged and expressed in 293 cells. Nuclear extracts were subjected to peptide pulldowns as indicated and probed with the antibodies shown. (F) Recombinantly expressed and purified dual PHD3/Bromodomain of MLL1 binds specifically to H3 lysine 4 di and trimethyl peptides (top panel). A W1594E point mutation in PHD3 abolishes this interaction (bottom panel). See also Figure S4.