Figure 1. CaM-binding sites on the MA structure.
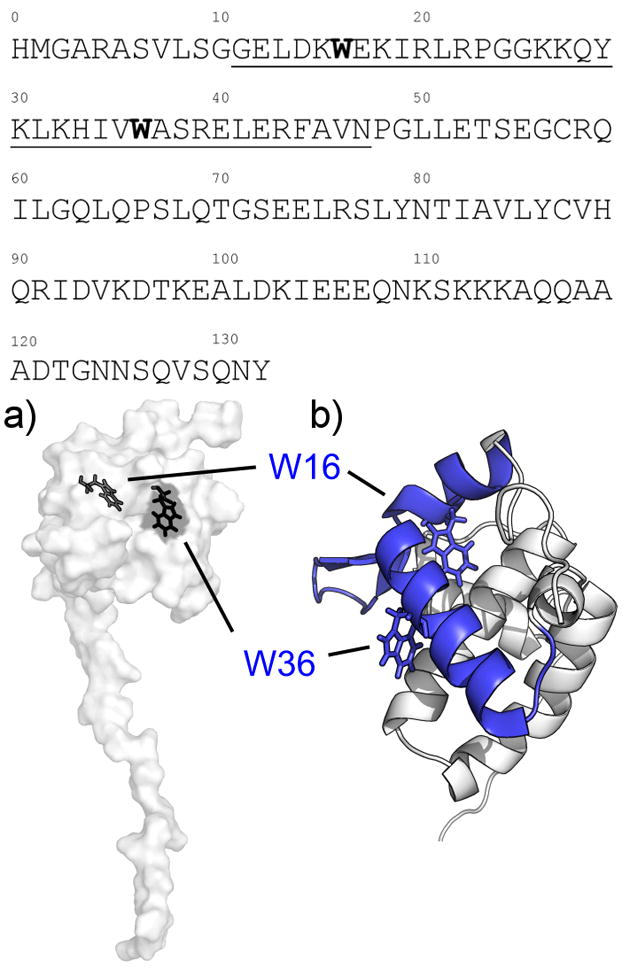
(Top) Amino acid sequence for the MA construct used in this experiment, depicting the proposed CaM-binding sites (underlined) and the tryptophan residues located within (bold). a) Surface depiction of the MA NMR structure (PDB: 2HMX) showing the tryptophan residues (black) located within the N-terminal lobe of MA, with W16 completely buried within the hydrophobic core while W36 is partially exposed to solvent. b) Closeup view of the N-terminal globular domain of MA and the CaM-binding site (blue) in cartoon form. The site is composed of two short α-helices connected by a basic loop region.
