Abstract
In adults, irritable bowel syndrome (IBS) and functional dyspepsia (FD) are chronic conditions that often start during childhood. We investigated mucosal serotonin (5-HT) signalling in children with the idea that data from subjects with a shorter history may improve our understanding of underlying pathophysiological mechanisms.
Methods
98 children undergoing gastroscopy or colonoscopy were prospectively studied. Biopsy specimens were evaluated for inflammation, enterochromaffin cell numbers, 5-HT content, and mRNA levels for the synthetic enzyme, tryptophan hydroxylase 1 (TpH1) and the serotonin transporter (SERT) were assessed by quantitative real-time RT-PCR.
Results
Data from 12 children with IBS and 17 with FD were compared to age-matched controls (12 with rectal biopsies and 12 with gastric biopsies) and to subjects with organic disorders. In patients with FD, a small number of immune cells were observed in the gastric mucosa in half of the patients, but no abnormalities with respect of the 5-HT pathway were identified. In patients with IBS, no differences were detected between patients and controls regarding intraepithelial lymphocytes and CD3+ cells in the lamina propria although all patients exhibited at least a slight inflammatory infiltrate. In the IBS samples, higher 5-HT content (P<0.01) and lower SERT mRNA (P<0.05) were detected as compared to controls. Severe inflammation in the colonic mucosa had a high impact on 5-HT signalling with a significant decrease in EC cells (P<0.01) and 5-HT content (P<0.01) and a high SERT mRNA expression (P<0.01).
Conclusion
These results confirm in children the role of 5-HT signalling in IBS and argue against such a role in FD.
Keywords: Children, Abdominal pain, Irritable bowel syndrome, Functional Dyspepsia Serotonin, Enterochromaffin cells, Serotonin reuptake transporter, Visceral sensitivity
Introduction
Functional gastrointestinal disorders (FGID) are defined as recurrent symptoms unexplained by structural or biochemical anomalies. Fifteen percent of school age children are affected by abdominal pain secondary to FGID.1
Irritable bowel syndrome (IBS) and functional dyspepsia (FD) as defined by the Rome III criteria are the most frequent painful FGID in children. 2 The pathophysiology of FGID is not clearly understood; however, since 2001, several different groups 3-7 have reported that rectal hypersensitivity is found in 75% to 100% of children with IBS, although, in adults, its prevalence varies from 20% 8 to 94% 9 across studies. This suggests (i) that visceral hypersensitivity is a more reliable marker in children than in adults, and (ii) that in adults the chronic conditions with symptoms persisting for years could affect and alter the initial pathophysiological mechanisms. In that context, studying young patients, with a shorter history than adults may increase the possibility of demonstrating the role of underlying pathophysiological mechanisms.
Altered mucosal serotonin (5-HT) signalling is one of several hypothetical mechanisms underlying functional gastrointestinal disorders. 5-HT is synthesized by enterochromaffin (EC) cells, which utilize tryptophan hydroxylase-1 (TpH-1) as their rate-limiting enzyme in the biosynthesis of 5-HT.10 5-HT is released locally and acts on specific receptors located on nearby nerve fibers and cells in the lamina propria. The 5-HT selective reuptake transporter (SERT), terminates the actions of 5-HT by removing it from the interstitial space.11 Organic cation transporter-1 (OCT-1) is a low affinity 5-HT transporter and is expressed in human GI tract.12 OCT-1 is thought to contribute to the inactivation of 5HT when SERT is absent or deficient.13, 14 Studying 5-HT signalling in the intestinal mucosa in children with FGID is of importance for several reasons : (i) 5-HT plays a critical role in the regulation of gastrointestinal motility, secretion, and sensation through specific receptors that are widely expressed within the intrinsic primary afferent neurons, on smooth muscle cells, enterocytes, and extrinsic afferent nerve fibers.10; (ii) Changes in 5-HT signalling have been reported in blood15, 16 and colonic mucosa17, 18 of adults with IBS with conflicting results for the latter; (iii) despite the withdrawal of tegaserod and alosetron, both acting on 5-HT4 and 5-HT3-receptors which were shown beneficial in IBS in adults, several drugs with an action on the 5-HT signalling, such as 5-HT2b-receptor antagonist or TpH-1 inhibitor and potentially useful in the treatment of IBS are under development.19, 20
Taking advantage of studying young patients with a short duration of symptoms, this study was designed to test the hypothesis that, in children with IBS or FD, 5-HT signalling is altered in the digestive mucosa.
Methods
Subjects
Gastric or colonic tissues samples were prospectively obtained from children aged 8 to 18 years for whom a colonoscopy or gastroscopy was required in their evaluation. Potential subjects were excluded from the study if they had an acute intestinal infection (acute gastroenteritis) during the 4 weeks preceding the exam. The day of the procedure, all children filled a validated questionnaire, the Questionnaire on Pediatric Gastrointestinal Symptoms in Children (QPGS), which evaluates gastrointestinal symptoms and was adapted to the Rome III diagnostic criteria of FGID in children.21, 22
Six gastric (corpus) and/or colonic (rectal) biopsies were obtained in mucosa free of ulcerative or aphtous lesions and each sample was immediately placed into a prepared Eppendorf tube, weighed, and processed for histology, immunohistology, 5-HT content dosage and RNA extraction.
All patients were reassessed 3 months after the procedures to determine their final diagnosis. The final diagnosis of each participant was established by studying the patient file and if necessary in collaboration with the patient's physician after examination of the QPGS. Participants were classified in one of the 2 following groups:
Patients with IBS or FD according to Rome III criteria and
Subjects with non functional (organic) GI disorder.
According to the responses regarding the symptoms provided in the QPGS (in which abdominal pain was reported or not) the subjects with non functional GI disorders were further divided into:
Subjects with painful non functional GI disorder and
Subjects with non-painful non functional GI disorder.
The protocol was approved by the institutional ethics committee and appropriate consent was obtained from all participants; consent was signed by the parents or legal guardian and by the child himself/herself if 14 years or older prior to the procedure.
Measure of 5-HT content in gastric or rectal mucosal
The 5-HT content in homogenized biopsy specimen from each individual was analyzed using an enzyme immunoassay kit according to the manufacturer's instructions (Beckman Coulter, Mississauga, ON).
Inflammation Assessment
Inflammation is known to influence 5-HT signalling23; therefore, the mucosal inflammatory condition was determined for each individual included in the study. H&E-stained tissue 4 μm-thick paraffin sections from each individual in the study were blindly assigned an inflammatory activity score on a scale of 0-3 by an experienced pathologist (N.P.). The scoring system was as follows: 0, normal numbers of inflammatory cells in the lamina propria with no active inflammation; 1, minimally increased numbers of inflammatory cells in the lamina propria and /or a rare neutrophil/eosinophil with the crypt epithelium; 2, mild increased numbers of inflammatory cells in the lamina propria and/or mild active inflammation; 3, significantly/severely increased numbers of inflammatory cells in the lamina propria and/or moderate/severe active inflammation. Score of 0 and 1 were considered to be within the range of normal. Scores 2 and 3 were considered to be histopathologically inflamed. In order to further characterize the inflammatory infiltrate in the digestive mucosa of children with FGID, we quantified lymphocytes, neutrophils and eosinophils in the colonic and gastric mucosa of patients with FD and IBS and compared them to controls. Cells in the lamina propria were assessed as the total number of inflammatory cells within three to five non-overlapping high power fields at 40 × magnification, with H&E staining. Cells were classified as lymphocytes, neutrophils or eosinophils according to their typical morphology and staining properties. Fields containing lymphoid aggregates were excluded. Lymphocytes and intraepithelial lymphocytes were counted as CD3 positive cells.
Immunohistochemistry
One biopsy from each subject was fixed 2-3 hours in 2% paraformaldehyde / 0.2% picric acid and transferred in 70% ethanol at 4°C. Immunohistochemistry techniques were performed on an automate (NextES IHC, Ventana) and indirect immunoperoxidase staining was performed (See Supplementary Methods).
Lymphocytes were counted in the lamina propria respectively as CD3 positive cells within three to five non-overlapping high power fields at 40× magnification. Intraepithelial lymphocytes were enumerated as CD3 positive cells per 100 epithelial cells. Numbers of enteroendocrine cells (chromogranin A immunoreactive) and EC cells (serotonin immunoreactive) were evaluated at the 40× magnification on the entire sections using a quantitative score (number of positive cells divided by number of glandular epithelial cells). All counts were performed blindly by an experienced pathologist (N.P.)
Measurement of RNA
One specimen from each individual was placed in a tube containing RNAlater™ (Qiagen). Reverse Transcription and quantitative real-time polymerase chain reaction was performed for serotonin transporter (SERT), organic cation transporters (OCT1) and tryptophan hydroxylase (Tph1) in each sample were normalized by geometric mean of three reference genes (18S, Phosphomannomutase 1 and hypoxanthine phosphoribosyl-transferase 124 (See Supplementary Methods).
Statistical analyses
Summary data are expressed as means (± standard deviation) of normally-distributed data and medians (25th-75th percentile) for non-normally-distributed data. N-values represent the number of subjects included in the data set. Comparisons between the patients (FD or IBS) and the controls used the Student t test or Mann Whitney U test to compare means or medians respectively and the χ2 test for count data. A one-way ANOVA with a Tukey post-hoc test was used to compare values from inflammed biopsies obtained from subjects with organic disorders and values from non-inflammed biopsies obtained from individuals with organic disorders or controls. Spearman's test was used to correlate the different variables. Significance was expressed at the P <0.05 level.
Results
Patients
One hundred and eighteen subjects were recruited for the study (Supplementary Figure 1). Among them, specimens were not collected in 6 for technical reasons (ulcerations or aphtous lesions in the mucosa). In fourteen participants who underwent endoscopy, the diagnosis of FGID was performed but neither IBS nor FD was confirmed by the Rome III criteria; these subjects were therefore excluded from the analysis. Ninety-eight children (42 boys, mean age 13.9 years range 8 to 18) were finally included for the study. Fifty-one colonic and fifty-seven gastric specimens were collected. Subject demographics and diagnoses are summarized in the Tables 1 and 2. Although female patients were more numerous in the FGID group, there was no difference regarding the sex ratio between patients and control subjects (χ2 test; P>0.05). All patients with IBS and FD fulfilled the Rome III criteria. Although the pediatric Rome III criteria do not separate the different subtypes of IBS, we classified the patients according to their predominant alteration of transit as reported in the QPGS: 10 patients reported diarrhea predominant IBS and 2 alternating IBS. Supplementary Table 1 provides symptoms severity assessed by QPGS at the time of investigation. All patients with IBS underwent colonic biopsies and all patients with FD gastric biopsies.
Table 1.
Demographics and diagnoses of the 57 subjects who underwent a gastroscopy
| Diagnosis | Number | Sex M/F | Age (years; median, range) | Inflammation grading |
|---|---|---|---|---|
| Functional dyspepsia | 17 | 7/10 | 14 (10-17) | (0) : 7 |
| (1) : 9 | ||||
| (2) : 1 | ||||
| Controls | 40 | 18/22 | ||
| Non painful GI disorder | 19 | 9/10 | 14 (8-18) | (0) : 7 |
| Eosinophilic esophagitis/GER | 7 | (1) : 9 | ||
| Polyps screening/normal gastroscopy | 5 | (2) : 2 | ||
| Crohn's disease | 4 | (3) : 1 | ||
| Celiac disease | 3 | |||
| Painful GI disorder | 21 | 9/12 | 14 (8-18) | (0) : 6 |
| Eosinophilic esophagitis/GER | 8 | (1) : 10 | ||
| Crohn's disease | 9 | (2) : 5 | ||
| Gallblader lithiasis | 1 | |||
| Celiac disease | 1 | |||
| Helicobacter pylori gastritis | 2 | |||
Table 2.
Demographics and diagnoses of the 51 subjects who underwent a colonoscopy
| Diagnosis | Number | Sex M/F | Age (years; median, range) | Inflammation grading |
|---|---|---|---|---|
| IBS | 12 | 3/9 | 14 (11-16) | (0) : 1 |
| (1) : 11 | ||||
| Controls | 39 | 19/20 | ||
| Non painful GI disorder | 24 | 12/12 | 14 (8-18) | (0) : 4 |
| Polyps screening/normal colonoscopy | 14 | (1) : 13 | ||
| Crohn's disease | 10 | (2) : 5 | ||
| (3) : 2 | ||||
| Painful GI disorder | 15 | 7/8 | 14 (8-18) | (0) : 3 |
| Crohn's disease | 15 | (1) : 6 | ||
| (2) : 1 | ||||
| (3) : 5 | ||||
Controls
Patients were compared to age-matched subjects with non functional GI disorder in whom colonic or gastric biopsies were obtained. These subjects were divided into 2 groups: those reporting no painful symptoms and those with painful complaints as reported in the questionnaires. Specimens (gastric biopsies n= 12 [5 males and 7 females]; colonic biopsies n=12 [7 males and 5 females]) from subjects whose diagnosis was not an inflammatory bowel disease or a celiac disease and, who reported no painful complaints, and for whom the analysis of the specimens did reveal an inflammation grade of 0 or 1 were included in the control group. The others were considered as subjects with organic diseases. Because gastric specimens from subjects with non functional GI disease were predominantly non inflamed (Table 1), the number of gastric biopsies with inflammation grading ≥ 2 was too low to perform statistical analysis. The results obtained with these 8 biopsies were not included in the analysis.
Inflammation in IBS and FD
Analysis of the inflammatory condition of biopsy specimens collected in children with IBS showed that 11/12 patients had a minimal inflammation (grade 1) and the remaining specimen had a complete absence of inflammation (grade 0)(Table 2). In the stomach, the proportion was slightly different with biopsies from 7/17 FD patients having no inflammation (grade 0), 9/17 exhibiting minimal inflammation (grade 1), and 1/17 moderate inflammation (grade 2)(Table 1). No differences were detected between patients and controls regarding intraepithelial lymphocytes and CD3+ cells in the lamina propria (Table 3) although all patients exhibited at least a slight inflammatory infiltrate in the lamina propria. Neutrophils were not detected in sections from any of the subjects in any of the groups.
Table 3.
Characterization of mucosal inflammation in patients with IBS or FD
| IBS | Controls | IBS vs Controls | FD | Controls | FD vs Controls | |
|---|---|---|---|---|---|---|
| IEL | 6.0 (4.1 – 14.6) | 6.1 (1.0 – 10.2) | NS | 8.5 (4.2 – 14.3) | 6.8 (3.2 – 10.3) | NS |
| Lymphocytes | 48.5 (18 – 126) | 44.2 (29 – 104) | NS | 43.7 (20 -60) | 49 (17 -87) | NS |
| Neutrophils | 0.0 (0 – 2) | 0.0 (0 – 1) | NS | 0.0 (0 – 1) | 0.0 (0 – 1) | NS |
| Eosinophils | 0.0 (0 – 0) | 0.0 (0 – 1) | NS | 0.0 (0 – 0) | 0.0 (0 – 0) | NS |
Data are expressed as median and range. Intraepithelial lymphocytes are expressed per 100 epithelial cells and lymphocytes, neutrophils and eosinophils as the number of cells per high power field.
IBS: irritable bowel syndrome; FD : functional dyspepsia; IEL : intraepithelial lymphocytes
Enterochromaffin cells and 5-HT immunoreactive cells
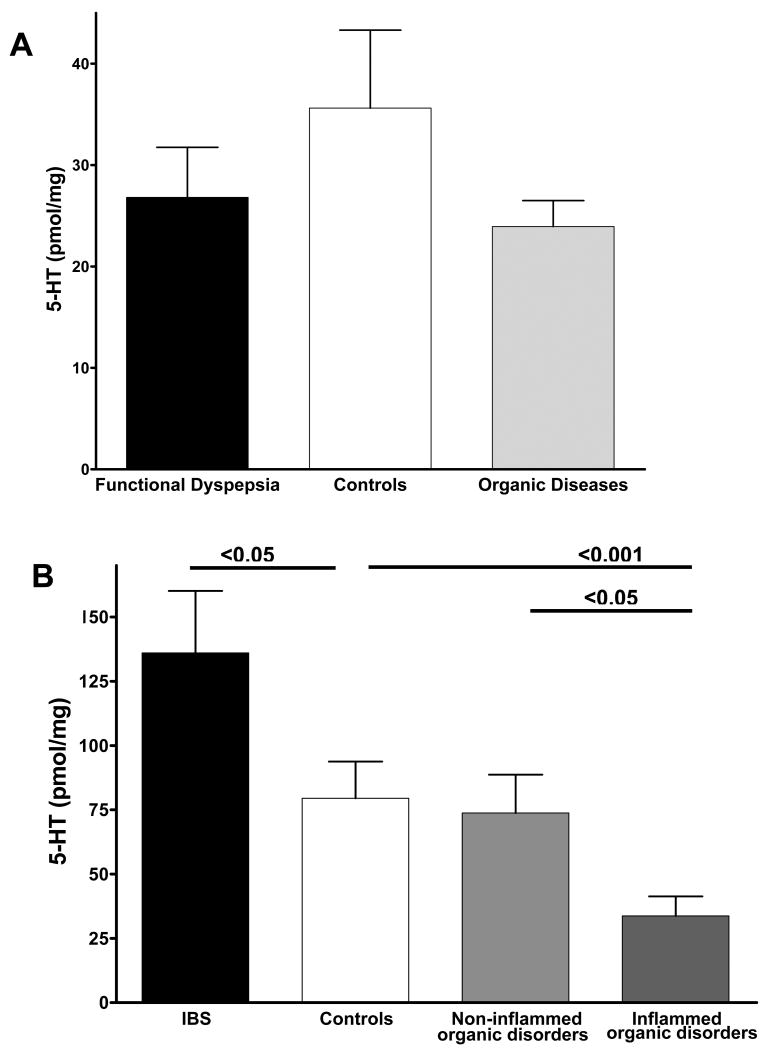
In patients with FD, enteroendocrine (chromogranin positive) cell and EC (5-HT positive) cell counts in the gastric mucosa were similar to those of controls and subjects with organic disease (Figure 1A and 1B).
Figure 1.
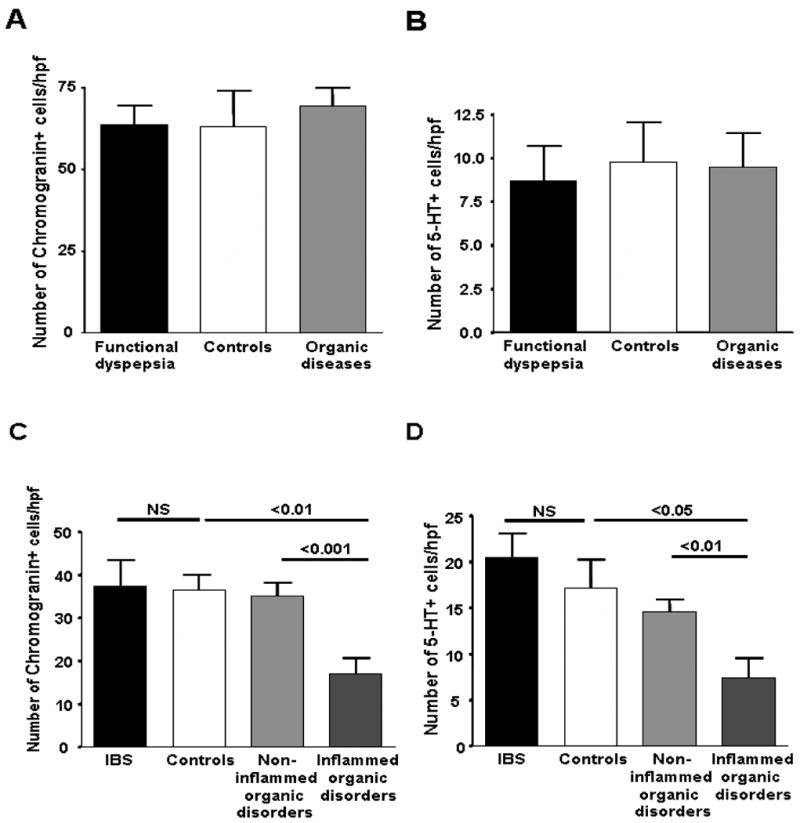
(A) Enteroendocrine (chromogranin positive) cell and (B) enterochromaffin (5-HT positive) cell counts in the gastric mucosa of patients with functional dyspepsia (FD) are similar as compared to control subjects and subjects with organic diseases. (C) Enteroendocrine (chromogranin positive) cell and (D) enterochromaffin (5-HT positive) cell counts in the rectal mucosa of patients with irritable bowel syndrome (IBS), control subjects and subjects with organic diseases with inflammation grading ≥ 2 (Inflammed organic disorders) and without inflammation (Non-inflammed organic disorders). No significant differences were detected in enteroendocrine and enterochromaffin cell counts of samples from patients with IBS as compared to controls. Biopsies with inflammation grading ≥ 2 obtained from subjects with organic disease exhibited significant lower enteroendocrine and enterochromaffin cell counts as compared to controls and biopsies without inflammation (grade ≤ 1).
In patients with IBS, enteroendocrine cell counts in the colonic mucosa were comparable to those of controls. However, the presence of severe inflammation (grade ≥ 2) in specimens of subjects with organic disease led to a significant decrease of chromogranin immunoreactive cells as compared to controls and to subjects with non-inflammed organic diseases (Figure 1C). A similar pattern was detected for EC cell counts (Figure 1D).
Serotonin content
In patients with FD, the 5-HT content of the gastric mucosa was similar to controls and to subjects with organic disease (Figure 2A).
Figure 2.
(A) Serotonin (5-HT) content in the gastric mucosa of patients with functional dyspepsia (FD) is similar as compared to control subjects and subjects with organic diseases. (B) 5-HT content in the rectal mucosa of patients with irritable bowel syndrome (IBS), control subjects and subjects with organic diseases with inflammation grading ≥ 2 (Inflammed organic disorders) and without inflammation (Non-inflammed organic disorders). 5-HT content is significantly higher in the rectal mucosa of patients with IBS as compared to control subjects. 5-HT levels are significantly lower in biopsies obtained from subjects with Inflammed organic disorders as compared to controls and biopsies from Non-inflammed organic disorders.
In patients with IBS, 5-HT content of the rectal mucosa was higher than controls (Figure 2B). Inflammation appeared to influence the 5-HT content in the rectal mucosa as significantly lower 5-HT concentrations were detected in the inflamed specimens from subjects with organic disease than controls and non-inflamed organic diseases (Figure 2B).
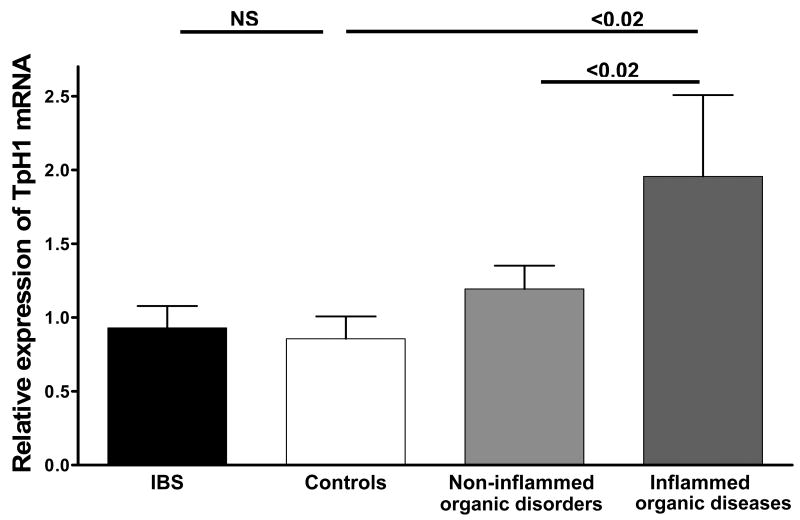
TpH-1 mRNA
TpH-1 is the rate limiting enzyme of 5-HT synthesis in EC cells.10 In the patients with FD, transcript levels for TpH-1 in gastric biopsies were similar to those measured in controls (median and interquartile range of relative expression of TpH-1 for FD = 5.65; 2.5 – 7.1 versus 2.67; 1.62 – 3.7 for controls; P>0.05).
In patients with IBS there was no difference between patients and controls and with non-inflamed controls (Figure 3). However, the presence of severe inflammation (grade ≥ 2) in specimens of subjects with organic disease led to a significant overexpression of TpH-1 mRNA as compared to subjects with non-inflammed organic diseases (Figure 3), suggesting that inflammation itself increases TpH-1 mRNA expression.
Figure 3.
Relative expression of tryptophan hydroxylase-1 (TpH-1) mRNA in the rectal mucosa of patients with irritable bowel syndrome (IBS), control subjects and subjects with organic diseases with inflammation grading ≥ 2 (Inflammed organic disorders) and without inflammation (Non-inflammed organic disorders). No significant differences were detected in TpH-1 transcript levels of samples from patients with IBS as compared to controls. TpH-1 transcript levels are significantly higher in biopsies obtained from subjects with Inflammed organic disorders as compared to controls and biopsies from Non-inflammed organic disorders.
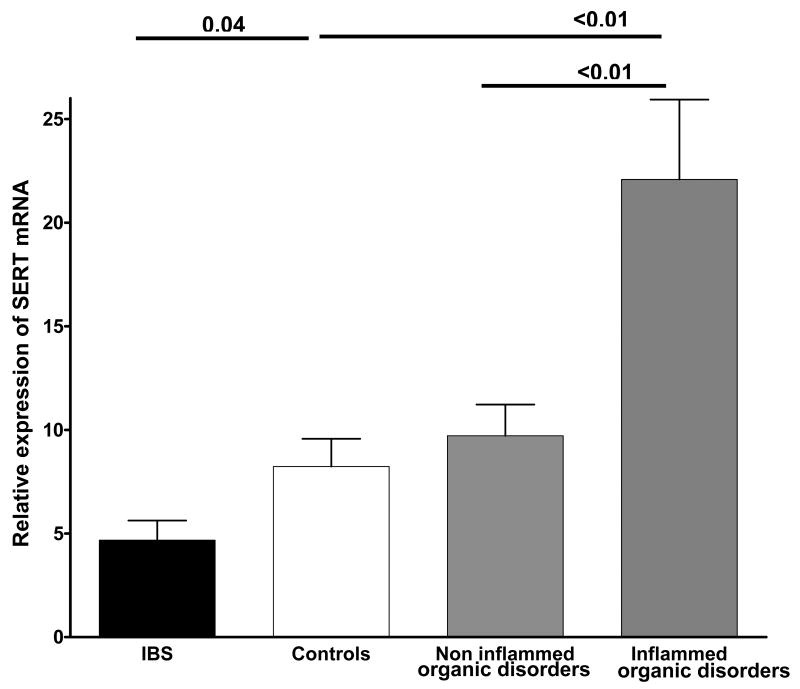
SERT mRNA
In patients with FD, SERT mRNA expression in the gastric specimens was comparable to controls and subjects with organic diseases (median and interquartile range of relative expression of SERT for FD = 2.49; 1.93 – 3.7 versus 2.67; 1.97 – 3.1 for controls; P>0.05).
In rectal mucosa of the patients with IBS, the SERT mRNA level was significantly lower than in controls (Figure 4). The presence of severe inflammation (grade ≥ 2) in specimens of subjects with organic disease led to a significant overexpression of SERT mRNA as compared to subjects with non-inflammed organic diseases (Figure 4).
Figure 4.
Relative expression of serotonin reuptake transporter (SERT) mRNA in the rectal mucosa of patients with irritable bowel syndrome (IBS), control subjects and subjects with organic diseases with inflammation grading ≥ 2 (Inflammed organic disorders) and without inflammation (Non-inflammed organic disorders). A significantly lower level of SERT transcript was detected in samples from patients with IBS as compared to controls. SERT transcript levels are significantly increased in biopsies obtained from subjects with Inflammed organic disorders as compared to controls and biopsies from Non-inflammed organic disorders.
OCT-1 mRNA
Because OCT-1 contributes to the inactivation of 5-HT in the absence of SERT, we examined OCT-1 mRNA expression to further find a mechanism to explain the higher content in 5-HT in IBS and to explore a possible involvement of 5HT signalling in FD. No difference was seen in the gastric mucosa of patients with FD as compared to controls either inflamed or not (median and interquartile range of relative expression of OCT-1 for FD = 1.23; 0.95 – 1.51 versus 1.41; 1.13 – 1.64 for controls; P>0.05).
Similarly, OCT-1 mRNA expression was identical in the rectal mucosa of patients with IBS and controls and subjects with organic disease with or without inflammation (Supplementary Figure 2).
Correlation between SERT mRNA and 5-HT content
A significant inverse correlation was also found between SERT mRNA and 5-HT content in the colonic specimens (r=-0.47; P=0.0003)(Supplementary Figure 3). No such correlation was found in the gastric specimens.
Discussion
IBS and FD are highly prevalent in children, but as in adults, the pathophysiology is not yet fully understood. Prior to the current study, no data were available on mucosal abnormalities in FGID in children. We conducted a prospective study to test the hypothesis that mucosal abnormalities are present in children with FD and IBS. We designed our study protocol in such a way that we were able to analyze not only controls with non functional GI (organic) disorders, but also “ideal” controls for which endoscopic procedures were performed for conditions not related to inflammatory and/or painful GI disorders such as colonic polyps screening.
FGID in adults are a chronic condition with symptoms that, in a majority of the cases, have begun during childhood, and persist for years.25-27 They can therefore be considered as a continuum from childhood to adulthood. One can suggest that the protracted evolution could affect and alter the initial pathophysiological mechanisms in such a way that a clear demonstration of involvement of 5-HT signalling is not easy and may explain the discrepancies in the literature.17, 18 In that context, studying young patients, with a shorter history than adults increases the possibility of demonstrating the pathophysiological mechanisms as illustrated by the higher prevalence of rectal hypersensitivity in children with IBS across studies from several independent groups3-7 than in adults.8, 9, 28
In patients with FD, a low inflammation grade in the gastric mucosa was detected in half of the patients, but no abnormalities with respect of the 5-HT pathway were identified as compared to controls. To our knowledge this study is the first to report data on 5-HT pathway in FD. Our data argue against a role of mucosal 5-HT in the symptoms of FD and are consistent with the lack of efficacy of pharmacological 5-HT signalling modulation in such patients.10 Other authors similarly failed to show any role of altered mucosal 5-HT biosynthetic and uptake capacity in upper abdominal symptoms in idiopathic gastroparesis.29 However, all gastric specimens evaluated in the present study were obtained from the corpus, a region known to have a low number of EC cells. The paucity of these cells may hamper the detection of any potential change occurring in FD or organic disorders relative to controls. Whether similar results may be obtained in other regions of the upper GI tract is debatable since for example SERT, TpH-1 and EC cells vary significantly in the different regions of the stomach and in the duodenum.30
In patients with IBS, we detected immune cells in the rectal mucosa in the majority of the cases and a higher availability of 5-HT with higher 5-HT content and lower SERT mRNA than in control subjects. This study provides for the first time data on inflammation and involvement of the mucosal 5-HT signalling pathway in pediatric IBS. Low-grade inflammation has been reported in the enteric ganglia31 and in the mucosa31, 32 of adult patients with IBS and recent studies outline the key role of the mucosal inflammation in driving possibly local modifications promoting peripheral sensitization in adults.33-35 The role of a GI infectious episode prior to the IBS symptoms was not specifically assessed in the patients included in the present study because of the major recall bias of such retrospective question.36, 37
We did not find a significant difference in enteroendocrine cells and EC cells between IBS and controls similar to previous adult studies17, 38 However, we found in the IBS patients a higher mucosal 5-HT availability than in controls. This higher availability was not due to a higher synthesis rate as shown by the normal mRNA expression of TpH-1 the rate limiting enzyme of 5-HT synthesis in EC cells. However, recent data demonstrate that TpH-2, the “brain” isoform of TpH, is also present in enteric neurons and may participate to 5-HT synthesis in the GI tract.39 Because we used mucosal biopsies that are superficial and do not contain submucosal or myenteric neuronal bodies, TpH-2 mRNA was not quantifiable and we can not exclude the possibility that the higher 5-HT mucosal content in IBS patients may not be related to higher TpH-2 expression. One possible mechanism to explain the higher 5-HT availability is the lower SERT mRNA expression in IBS rectal mucosa found in our population. The significant correlation found between 5-HT content and SERT mRNA in the colonic specimens highly reinforces this possible explanation. The low expression of SERT mRNA in IBS patients reported here was not due to a differential expression of the reporter genes as compared to the controls. Moreover, due to discrepancies in the literature, we paid particular attention to the quantification of the mRNA and used, as suggested in the most recent recommendations, 3 reporter genes and their geometrical mean. SERT mRNA expression in IBS has been previously reported in the adult literature with conflicting results. Coates et al. were the first to report a significant reduction of SERT mRNA expression in colonic mucosa of adult patients with IBS.17 The results were not replicated by Camilleri et al. 18 and Kerchkoffs et al. found conversely an increased expression of SERT mRNA in the duodenal mucosa of IBS patients.40 On the other hand, reports of elevated post-prandial plasma 5-HT concentrations in IBS-D16, 41 are consistent with a decreased capacity of the intestinal epithelial cells to take up 5-HT. To further examine the 5-HT uptake capacity in these samples, we studied the expression of OCT-1 mRNA, which transports 5-HT when SERT is absent. We did not find any difference between the patients and the controls suggesting that OCT-1 is neither defective nor overexpressed in the conditions examined here. SERT gene polymorphisms, which may be associated with diarrhea predominant IBS in adult females42, could also be involved in the modulation of 5-HT signaling in children with IBS.
In the present study, we report that colonic mucosal inflammation has a high impact on 5-HT signalling in children. Despite a high TpH-1 mRNA expression, we report low 5-HT availability in the subjects with a high grade inflammation in the rectal mucosa with a significant decrease in EC cells and 5-HT content and a high SERT mRNA expression. The low 5-HT content is not due to a loss of tissue as the specimens were not taken into ulcerative lesions; the high SERT mRNA expression is not related to low expression of reporter genes as previously mentioned.
All patients with a high inflammatory grade were afflicted by Crohn's disease. Recent animal studies have demonstrated that 5-HT plays a key role in inflammation 43, 44 but conflicting results have been reported in inflammatory colitis with some authors reporting increased EC cells and 5-HT content45, 46 when other reported a decrease in EC cells and 5-HT.17, 47-49 Our present data are in keeping with the work of Motomura et al. showing that Th1 response, that characterizes Crohn's disease, has been shown to provoke a decrease of EC cells and 5-HT availability.50 Elevated SERT transcripts in inflammation was an unexpected finding since decreased SERT mRNA levels have been reported in ulcerative colitis17 and in animal models of inflammatory colitis.51 On the other hand, Minderhound et al. did not find any difference in SERT mRNA neither in the ileum nor in the colon in patients with Crohn's disease as compared to controls52 and a recent large study reports that SERT mRNA was significantly higher in the ileal mucosa of patients with Crohn's disease.53 Moreover, our data on SERT mRNA are consistent with the lower 5-HT content measured in the inflamed colonic biopsies. The exact role played by 5-HT and the regulation of its synthesis and uptake during the course of inflammation and the influence of anti-inflammatory drugs are unknown. The apparent discrepancy between the high level of TpH-1 mRNA and the low 5-HT content reported here requires further studies.
Potential limitations of this study are related to the relatively low number of patients included. However, despite the difficulties to obtain tissue specimens in children, we were able to recruit children who can be considered as appropriate controls since they were asymptomatic regarding painful symptoms and were investigated for non painful and non inflammatory GI disorders. Their specimens were carefully controlled for inflammatory status. We are also aware that the participants were recruited from a tertiary pediatric centre and therefore, those with IBS and FD may be at the more severe end of the spectrum of the functional disorders. If this is related to increased involvement of 5-HT signalling, it may result in an overestimation of the difference in IBS patients and controls but argues definitely against the potential role of 5-HT in FD. However, we can not conclude that the present findings are valid for all pediatric patients with FGID in the community. Finally it should be noted that these results do not necessarily apply to constipated predominant IBS patients since the present study was not designed to specifically include constipated patients.
Because this study was conducted in children and adolescents for whom the functional symptoms are of shorter duration than adults we believe that the present results are of great value and definitely confirm the role of 5-HT in IBS and argue against such a role in FD. We are however aware that FGID in childhood could not be exclusively explained by a biological model but are rather the result as in adults of complex interactions between biological (5-HT signalling) and social, familial and psychological traits. These results provide important data that should further encourage the development of new treatments oriented toward a lower 5-HT availability in patients with IBS such as tpH-1 inhibitors54 and 5-HT uptake enhancer.20
Supplementary Material
Acknowledgments
We gratefully acknowledge the staff of the Division of Gastroenterology at Hôpital Sainte-Justine. We also thank Patricia Perrault, RN, for her technical assistance.
This study was supported by a grant from the Canadian Association of Gastroenterology/CIHR/Abbott (CF) and NIHDK 62267 (GM).
Abbreviations
- 5-HT
Serotonin
- EC
enterochromaffin cells
- FGID
Functional gastrointestinal disorders
- FD
Functional dyspepsia
- IBS
Irritable bowel syndrome
- OCT-1
Organic cation transporter-1
- QPGS
Questionnaire on pediatric gastrointestinal symptoms in children
- TpH-1
tryptophan hydroxylase-1
Footnotes
Christophe Faure designed the study. He obtained funds from the Canadian Association of Gastroenterology. He analyzed the data and wrote the manuscript.
Natalie Patey performed and analyzed all the pathological data.
Cindy Gauthier and Elice Brooks were involved in the acquisition of data and brought technical support for 5-HT content and RT-PCR experiments
Gary Mawe was involved in the study design and analysis of the data and critically revised the manuscript.
Conflict of interest: NONE
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Subcommittee on Chronic Abdominal Pain. Chronic Abdominal Pain in Children. Pediatrics. 2005;115:e370–381. doi: 10.1542/peds.2004-2523. [DOI] [PubMed] [Google Scholar]
- 2.Helgeland H, Flagstad G, Grøtta J, et al. Diagnosing Pediatric Functional Abdominal Pain in Children (4-15 Years Old) According to the Rome III Criteria: Results From a Norwegian Prospective Study. Journal of Pediatric Gastroenterology and Nutrition. 2009;49:309–315. doi: 10.1097/MPG.0b013e31818de3ab. [DOI] [PubMed] [Google Scholar]
- 3.Faure C, Wieckowska A. Somatic Referral of Visceral Sensations and Rectal Sensory Threshold for Pain in Children with Functional Gastrointestinal Disorders. J Pediatr. 2007;150:66–71. doi: 10.1016/j.jpeds.2006.08.072. [DOI] [PubMed] [Google Scholar]
- 4.Van Ginkel R, Voskuijl WP, Benninga MA, et al. Alterations in rectal sensitivity and motility in childhood irritable bowel syndrome. Gastroenterology. 2001;120:31–8. doi: 10.1053/gast.2001.20898. [DOI] [PubMed] [Google Scholar]
- 5.Iovino P, Tremolaterra F, Boccia G, et al. Irritable bowel syndrome in childhood: visceral hypersensitivity and psychosocial aspects. Neurogastroenterol Motil. 2009;21:940–e74. doi: 10.1111/j.1365-2982.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- 6.Di Lorenzo C, Youssef NN, Sigurdsson L, et al. Visceral hyperalgesia in children with functional abdominal pain. J Pediatr. 2001;139:838–43. doi: 10.1067/mpd.2001.118883. [DOI] [PubMed] [Google Scholar]
- 7.Halac U, Noble A, Faure C. Rectal Sensory Threshold for Pain is a Diagnostic Marker of Irritable Bowel Syndrome and Functional Abdominal Pain in Children. J Pediatr. 2009 doi: 10.1016/j.jpeds.2009.06.062. [DOI] [PubMed] [Google Scholar]
- 8.Camilleri M, McKinzie S, Busciglio I, et al. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772–81. doi: 10.1016/j.cgh.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mertz H, Naliboff B, Munakata J, et al. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 10.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Wade PR, Chen J, Jaffe B, et al. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci. 1996;16:2352–64. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24:1227–51. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- 13.Chen JJ, Li Z, Pan H, et al. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21:6348–61. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitt A, Mossner R, Gossmann A, et al. Organic cation transporter capable of transporting serotonin is up-regulated in serotonin transporter-deficient mice. J Neurosci Res. 2003;71:701–9. doi: 10.1002/jnr.10521. [DOI] [PubMed] [Google Scholar]
- 15.Atkinson W, Lockhart S, Whorwell PJ, et al. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2006;130:34–43. doi: 10.1053/j.gastro.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 16.Dunlop SP, Coleman NS, Blackshaw E, et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–57. doi: 10.1016/s1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 17.Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–64. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Camilleri M, Andrews CN, Bharucha AE, et al. Alterations in expression of p11 and SERT in mucosal biopsy specimens of patients with irritable bowel syndrome. Gastroenterology. 2007;132:17–25. doi: 10.1053/j.gastro.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camilleri M, Chang L. Challenges to the therapeutic pipeline for irritable bowel syndrome: end points and regulatory hurdles. Gastroenterology. 2008;135:1877–91. doi: 10.1053/j.gastro.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanger GJ. 5-Hydroxytryptamine and the gastrointestinal tract: where next. Trends Pharmacol Sci. 2008 doi: 10.1016/j.tips.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Rasquin A, Di Lorenzo C, Forbes D, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527–37. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drossman DA, Corazziari E, Delvaux M, Spiller R, Talley NJ, Thompson WG, Whitehea WE, editors. The functional gastrointestinal disorders : Rome III. Third. McLean, VA: Degnon Associates, Inc.; 2006. Rome III diagnostic questionnaire for the pediatric functional GI disorders; pp. 961–990. [Google Scholar]
- 23.Mawe GM, Coates MD, Moses PL. Review article: intestinal serotonin signalling in irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:1067–76. doi: 10.1111/j.1365-2036.2006.02858.x. [DOI] [PubMed] [Google Scholar]
- 24.Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chitkara DK, van Tilburg MA, Blois-Martin N, et al. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol. 2008;103:765–74. doi: 10.1111/j.1572-0241.2007.01722.x. quiz 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howell S, Poulton R, Talley NJ. The natural history of childhood abdominal pain and its association with adult irritable bowel syndrome: birth-cohort study. Am J Gastroenterol. 2005;100:2071–8. doi: 10.1111/j.1572-0241.2005.41753.x. [DOI] [PubMed] [Google Scholar]
- 27.Christensen MF, Mortensen O. Long-term prognosis in children with recurrent abdominal pain. Arch Dis Child. 1975;50:110–4. doi: 10.1136/adc.50.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouin M, Plourde V, Boivin M, et al. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122:1771–7. doi: 10.1053/gast.2002.33601. [DOI] [PubMed] [Google Scholar]
- 29.van Lelyveld N, Ter Linde J, Schipper M, et al. Serotonergic signalling in the stomach and duodenum of patients with gastroparesis. Neurogastroenterol Motil. 2008;20:448–55. doi: 10.1111/j.1365-2982.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- 30.van Lelyveld N, Ter Linde J, Schipper ME, et al. Regional differences in expression of TPH-1, SERT, 5-HT(3) and 5-HT(4) receptors in the human stomach and duodenum. Neurogastroenterol Motil. 2007;19:342–8. doi: 10.1111/j.1365-2982.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- 31.Tornblom H, Lindberg G, Nyberg B, et al. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972–9. doi: 10.1053/gast.2002.37059. [DOI] [PubMed] [Google Scholar]
- 32.Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–83. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- 33.Piche T, Saint-Paul MC, Dainese R, et al. Mast cells and cellularity of the colonic mucosa correlated with fatigue and depression in irritable bowel syndrome. Gut. 2008;57:468–73. doi: 10.1136/gut.2007.127068. [DOI] [PubMed] [Google Scholar]
- 34.Liebregts T, Adam B, Bredack C, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–20. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 35.Aerssens J, Camilleri M, Talloen W, et al. Alterations in mucosal immunity identified in the colon of patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:194–205. doi: 10.1016/j.cgh.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robin S, Klara G. Postinfectious Irritable Bowel Syndrome. Gastroenterology. 2009;136:1979–1988. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 37.Saps M, Pensabene L, Di Martino L, et al. Post-infectious functional gastrointestinal disorders in children. J Pediatr. 2008;152:812–6. 816 e1. doi: 10.1016/j.jpeds.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 38.Park JH, Rhee PL, Kim G, et al. Enteroendocrine cell counts correlate with visceral hypersensitivity in patients with diarrhoea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2006;18:539–46. doi: 10.1111/j.1365-2982.2006.00771.x. [DOI] [PubMed] [Google Scholar]
- 39.Neal KB, Parry LJ, Bornstein JC. Strain-specific genetics, anatomy and function of enteric neural serotonergic pathways in inbred mice. J Physiol. 2009;587:567–86. doi: 10.1113/jphysiol.2008.160416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerckhoffs AP, Ter Linde JJ, Akkermans LM, et al. Trypsinogen IV, serotonin transporter transcript levels and serotonin content are increased in small intestine of irritable bowel syndrome patients. Neurogastroenterol Motil. 2008;20:900–7. doi: 10.1111/j.1365-2982.2008.01100.x. [DOI] [PubMed] [Google Scholar]
- 41.Houghton LA, Atkinson W, Lockhart C, et al. Sigmoid-colonic motility in health and irritable bowel syndrome: a role for 5-hydroxytryptamine. Neurogastroenterol Motil. 2007;19:724–31. doi: 10.1111/j.1365-2982.2007.00943.x. [DOI] [PubMed] [Google Scholar]
- 42.Yeo A, Boyd P, Lumsden S, et al. Association between a functional polymorphism in the serotonin transporter gene and diarrhoea predominant irritable bowel syndrome in women. Gut. 2004;53:1452–1458. doi: 10.1136/gut.2003.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghia JE, Li N, Wang H, et al. Serotonin Has a Key Role in Pathogenesis of Experimental Colitis Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 44.Bischoff SC, Mailer R, Pabst O, et al. Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2009;296:G685–G695. doi: 10.1152/ajpgi.90685.2008. [DOI] [PubMed] [Google Scholar]
- 45.Bishop AE, Pietroletti R, Taat CW, et al. Increased populations of endocrine cells in Crohn's ileitis. Virchows Arch A Pathol Anat Histopathol. 1987;410:391–6. doi: 10.1007/BF00712758. [DOI] [PubMed] [Google Scholar]
- 46.El-Salhy M, Danielsson A, Stenling R, et al. Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242:413–9. doi: 10.1046/j.1365-2796.1997.00237.x. [DOI] [PubMed] [Google Scholar]
- 47.Ahonen A, Kyosola K, Penttila O. Enterochromaffin cells in macrophages in ulcerative colitis and irritable colon. Ann Clin Res. 1976;8:1–7. [PubMed] [Google Scholar]
- 48.Kyosola K, Penttila O, Salaspuro M. Rectal mucosal adrenergic innervation and enterochromaffin cells in ulcerative colitis and irritable colon. Scand J Gastroenterol. 1977;12:363–7. doi: 10.3109/00365527709180942. [DOI] [PubMed] [Google Scholar]
- 49.Magro F, Vieira-Coelho MA, Fraga S, et al. Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel disease. Dig Dis Sci. 2002;47:216–24. doi: 10.1023/a:1013256629600. [DOI] [PubMed] [Google Scholar]
- 50.Motomura Y, Ghia JE, Wang H, et al. Enterochromaffin cell and 5-hydroxytryptamine responses to the same infectious agent differ in Th1 and Th2 dominant environments. Gut. 2008;57:475–81. doi: 10.1136/gut.2007.129296. [DOI] [PubMed] [Google Scholar]
- 51.Linden DR, Foley KF, McQuoid C, et al. Serotonin transporter function and expression are reduced in mice with TNBS-induced colitis. Neurogastroenterol Motil. 2005;17:565–74. doi: 10.1111/j.1365-2982.2005.00673.x. [DOI] [PubMed] [Google Scholar]
- 52.Minderhoud IM, Oldenburg B, Schipper ME, et al. Serotonin synthesis and uptake in symptomatic patients with Crohn's disease in remission. Clin Gastroenterol Hepatol. 2007;5:714–20. doi: 10.1016/j.cgh.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 53.Wojtal KA, Eloranta JJ, Hruz P, et al. Changes in mRNA expression levels of solute carrier transporters in inflammatory bowel disease patients. Drug Metab Dispos. 2009;37:1871–7. doi: 10.1124/dmd.109.027367. [DOI] [PubMed] [Google Scholar]
- 54.Liu Q, Yang Q, Sun W, et al. Discovery and Characterization of Novel Tryptophan Hydroxylase Inhibitors That Selectively Inhibit Serotonin Synthesis in the Gastrointestinal Tract. J Pharmacol Exp Ther. 2008;325:47–55. doi: 10.1124/jpet.107.132670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.