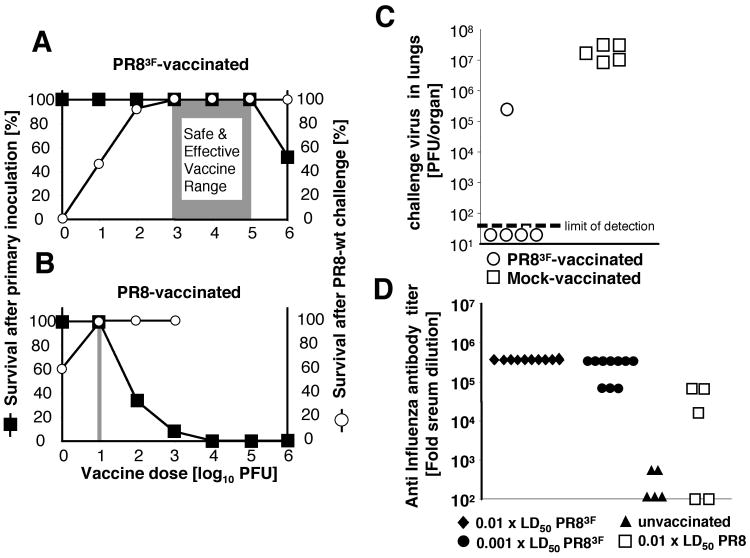
Fig. 3. Immune responses and protection.
(A, B) Vaccine Margin of Safety for PR8 wild type and deoptimized PR83F viruses. The left ordinate indicates the percentage of animals surviving the primary inoculation (black squares) with (A) PR83F or (B) wt PR8, at doses ranging between 100 to 106 PFU. After 28 days, the surviving, vaccinated animals were challenged with a single 1000 × lethal dose 50 of PR8 wild type virus. Disease and survival were monitored (right ordinate; open circles) for (A) PR83F- and (B) PR8-vaccinated mice. (C) Virus load in mouse lungs following wild type challenge of PR83F-vaccinated animals. 28 days following a single intranasal vaccination with 104 PFU PR83F mice were challenged with 1000 × LD50 of PR8 wt virus. Three days thereafter the level of challenge virus in lung homogenates was determined. (D) ELSIA determination of influenza-specific serum antibodies. 28 days after a primary infection, serum was collected, and anti-influenza IgG serum antibody titers were determined from animals that had received a primary inoculation of 0.01 × LD50 (black diamonds) or 0.001 × LD50 of PR83F (black circles), 0.01 × LD50 of PR8 (white squares), or saline (black triangles). ELISA antibody titer against PR8 virus antigen is expressed as the lowest reciprocal serum dilution that resulted in a positive ELISA signal (5 standard deviation above background).