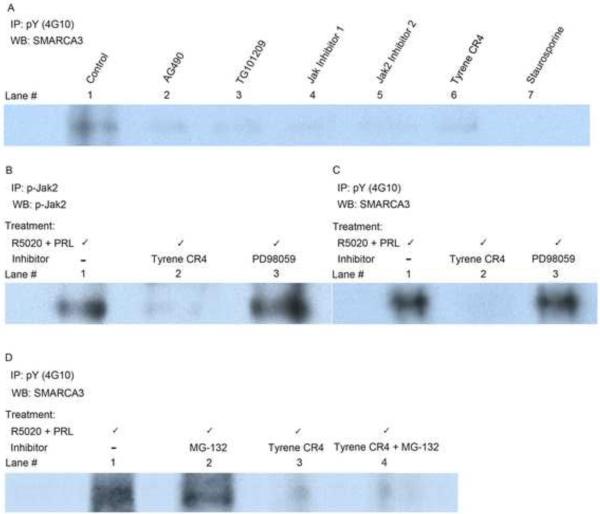
Figure 1.
Jak2 tyrosine phosphorylates RUSH in vivo.
Panel A, Prolactin-induced phosphorylation of RUSH was evaluated by Western analysis of whole cell extracts from serum-starved HRE-H9 cells treated with R5020 for 16–20 hours ± Jak2-specific inhibitors + prolactin for 2 minutes. This treatment of HRE-H9 cells applies to all panels in this figure.
Panel B. Inhibition of prolactin-induced Jak2 phosphorylation with Tyrene CR4 eliminated catalytically active Jak2 from nuclear extracts of HRE-H9 cells. Treatment with PD98059 had no effect.
Panel C. Inhibition of prolactin-induced Jak2 phosphorylation by Tyrene CR4 eliminated phospho-RUSH from nuclear extracts of HRE-H9 cells. Treatment with PD98059 had no effect.
Panel D. Nuclear phospho-RUSH was not degraded by the proteasome in vivo. MG-132 did not block a Tyrene CR4-mediated decrease in the level of phospho-RUSH from nuclear extracts of HRE-H9 cells. IP, Immunoprecipitation; WB, Western blot; pY (4G10), phosphotyrosine; p-Jak2, phospho-Jak2; SMARCA3, RUSH.