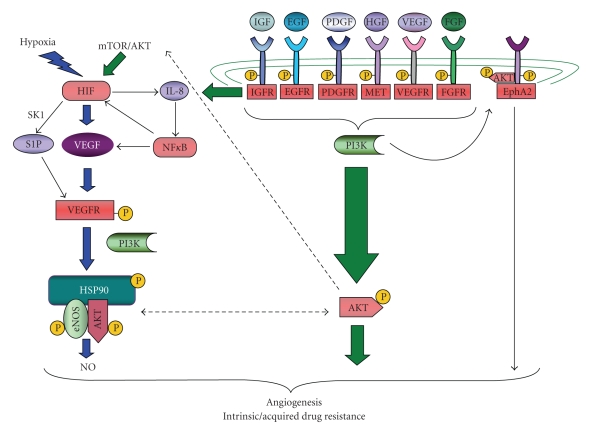
Figure 1.
Hsp90-dependent modulation of proangiogenic signaling pathways in cancer. Hsp90 regulates multiple arms of angiogenic signaling in cancer. Key signaling molecules that are either direct clients or indirectly modulated by Hsp90 are shaded in red. One pathway that is commonly upregulated during tumorigenesis is the HIF/VEGF signaling axis. Tumor hypoxia and other stimuli induce HIF expression and subsequent activity, leading to a cascade of events that reinforce VEGF expression and angiogenesis. Importantly, several key mediators of this pathway, including HIF and VEGFR, are dependent upon Hsp90 for their function. As indicated, RTK activation also potently upregulates HIF via AKT/mTOR -mediated translation. RTKs additionally transactivate EphA2, a recently identified Hsp90 client protein known to participate in tumor vascularization. Providing another level of complexity, HIF also upregulates the expression of several RTK ligands (e.g., HGF and TGF-alpha), as well as RTK receptors (EGFR, IGFR), thereby reinforcing these signaling networks. Hsp90 additionally plays a role in NFκB-dependent VEGF expression and regulates downstream effectors of VEGF signaling, including AKT-mediated eNOS phosphorylation. Given the intertwining levels among Hsp90 and angiogenic signaling cascades, Hsp90 intervention is predicted to impair signaling at many levels within these redundant pathways, with the overall effect of suppressing tumor angiogenesis.