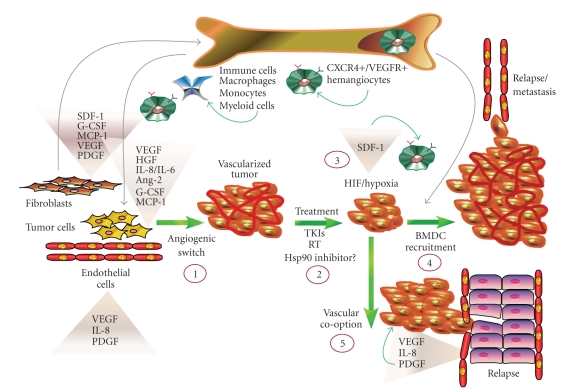
Figure 2.
Potential for Hsp90 intervention strategies in curtailing angiogenic processes. Tumor stromal cells, such as cancer-associated fibroblasts (CAFs) and endothelial cells (ECs), communicate with tumor cells via their secretion of cytokines and thus contribute to the angiogenic switch (1). Cytokines from recruited BMDC progenitors contribute to this milieu to further stimulate tumor vascularization (1). Hsp90 inhibition may prevent HIF-driven cytokine release from tumor and stromal cells (i.e., SDF-1, VEGF, HGF, etc.), as well as HIF-mediated CXCR4+ expression in BMDCs, with the potential effect of curtailing recruitment of CXCR4+ progenitors to the tumor. Hsp90 inhibition also attenuates cytokine signaling via RTK inhibition (i.e., VEGFR, PDGFR), which may collectively prevent or delay the angiogenic switch. Therapeutic approaches utilizing radiotherapy (RT), tyrosine kinase inhibitors (TKIs), or Hsp90-targeted agents suppress tumor vascularization and growth (2). This initial reduction in vascularity may promote tumor hypoxia (3), subsequent HIF activation, and SDF-1 secretion, the latter of which may further stimulate BMDC recruitment (4). Hsp90 inhibitors are similarly predicted to suppress BMDC recruitment and HIF-driven cytokine secretion as in (1). Alternatively, when challenged with reduced HIF expression and decreased BMDC recruitment, tumor cells may coopt the vasculature of normal tissue (5). In this scenario, Hsp90 suppression is predicted to reduce the efficiency of EC-derived factors that support this process. The overall efficacy of Hsp90 inhibition upon tumor vascularization will depend upon the balance of Hsp90-dependent and Hsp90-independent signaling effectors driving the angiogenic process.