Abstract
Atherosclerosis of native coronary arteries and graft arteriosclerosis in transplanted hearts are characterized by activation of innate and adaptive immune responses. Nucleic acids generated by infections or cell death have been detected within arteriosclerotic lesions and it is known that microbial and synthetic nucleic acids evoke inflammatory responses in cultured vascular cells. Here we report that model RNA, but not DNA, instigated robust cytokine and chemokine production from intact human coronary arteries containing both intrinsic vascular cells and resident/infiltrating leukocytes. A ssRNA analogue induced tumor necrosis factor-α and IP-10 secretion by isolated human PBMC, but not vascular cells. Conversely, synthetic dsRNA induced these inflammatory mediators by vascular cells, but not PBMC. IFN-γ, a cytokine linked to atherosclerosis and graft arteriosclerosis, potentiated the inflammatory responses of intact arteries and cultured vascular smooth muscle cells (VSMCs) to poly(I:C) and was necessary for inflammatory responses of VSMC to self-RNA derived from autologous cells. IFN-γ also induced the expression of TLR3, MDA5, and RIG-I dsRNA receptors. siRNA knockdown revealed that TLR3 mediated VSMC activation by poly(I:C), whereas MDA5 was more important for VSMC stimulation by self-RNA. IFN-γ-mediated induction of dsRNA receptors and priming for inflammatory responses to poly(I:C) was confirmed in vivo using immunodeficient mice bearing human coronary artery grafts. These findings suggest that IFN-γ, and by inference adaptive immunity, sensitizes the vasculature to innate immune activators, such as RNA and activation of IFN-γ-primed vascular cells by exogenous or endogenous sources of RNA may contribute to the inflammatory milieu of arteriosclerosis.
INTRODUCTION
Arteriosclerosis is a chronic inflammatory disease of the vessel wall that leads to pathological vascular remodeling, lumen loss, and ischemia. Coronary arteries are a common site of disease that occurs due to atherosclerosis in epicardial arteries of native (non-transplanted) hearts or from an accelerated alloimmune-driven vasculopathy (“graft arteriosclerosis”) in transplanted hearts. Both atherosclerosis and graft arteriosclerosis are characterized by activation of innate and adaptive immune responses with dysregulated production of cytokines (1, 2). Pro-inflammatory factors produced by artery-infiltrating macrophages and T cells, e.g. IFN-γ, activate intrinsic vascular cells, including endothelial cells (ECs) and vascular smooth muscle cells (VSMCs), to amplify and sustain vascular inflammation. Alloantigens may drive graft arteriosclerosis and it has been suggested that autoimmune responses driven by oxidized lipoproteins may underlie these pathological processes in atherosclerosis. Tissue injury may exacerbate both responses by augmenting adaptive immunity (3). Immune responses to pathogens may also play a role in accelerating the progression or triggering the complications of arteriosclerosis. Population-based studies have linked the occurrence of influenza virus infections to the incidence of myocardial infarctions (4) and cytomegalovirus has been implicated in the pathogenesis of graft arteriosclerosis as well (5). The mechanisms relating microbial infections to arteriosclerosis are poorly understood and it is possible that autoantigens contributing to vascular inflammation may be detected by similar means as foreign antigens by the immune system and intrinsic cells of the vessel wall.
Vascular cells and leukocytes in arteriosclerotic lesions express various pattern-recognition receptors (PRRs), including TLRs, that serve as the primary sensors for innate immunocytes and detect microbes by their pathogen-associated molecular patterns (PAMPs) (6). Viral and bacterial components that are recognized by host PRRs include nucleic acids, viz. single-stranded (ss)RNA, double-stranded (ds)RNA, and DNA rich in unmethylated CpG motifs. Specifically, ssRNA is detected by TLR7 and TLR8, dsRNA is detected by TLR3, retinoic acid-inducible gene I (RIG-I), and melanoma differentiation-associated gene 5 (MDA5), and CpG-rich DNA is detected by TLR9 (7). It has recently been shown that longer dsRNA is recognized by MDA5 and shorter dsRNA by RIG-I (8), and that cytosolic dsDNA without CpG residues elicits inflammatory responses (9). Although long dsRNA or cytosolic dsDNA is not normally found in host cells, nucleic acids released from damaged tissue or contained within endocytosed cellular remnants may serve as endogenous ligands for host PRRs (10, 11) and may cause or exacerbate disease (12, 13). Nucleic acids released by injured cells may be described as damage-associated molecular patterns (DAMPs), analogous to PAMPs. The localization of pathogen vs. host nucleic acids within the cell is an important determinant of immunostimulatory activity as TLR3, TLR7, TLR8, and TLR9 are largely localized in endosomes, whereas RIG-I and MDA5 are expressed in the cytoplasm. Binding of PAMPs (or DAMPs) to their respective PRRs activates multiple intracellular signaling cascades (7). Adapter molecules, including TIR domain-containing adaptor inducing IFN-β (TRIF) for TLR3, MyD88 for TLR7, TLR 8, and TLR9, and IFN-β promoter stimulator-1 (IPS-1) for RIG-I and MDA5, recruit signaling intermediaries leading to activation of transcription factors, such as interferon regulatory factor (IRF)3, IRF7, and NF-κB. The production of type-I IFNs and other pro-inflammatory mediators, e.g. TNF-α, IL-1β, IL-6, and IFN-γ-induced protein of 10 kDa (IP-10, also designated as CXCL10) play important roles in host defense against pathogens, but uncontrolled inflammatory responses may result in pathological conditions.
Given that arteriosclerosis is characterized by a pro-inflammatory milieu (1, 2), is associated with RNA and DNA virus infections (4, 5), and that degraded or oxidized host RNA and DNA accumulate within arterial plaques (14, 15), we investigated the role of exogenous and endogenous nucleic acids as triggers of inflammation within the arterial wall. Since it is known that microbial and synthetic nucleic acids evoke inflammatory responses in cultured vascular cells (16, 17) and since vascular cells within arteriosclerotic lesions show evidence of activation by IFN-γ, we examined if the pro-arteriosclerotic Th1 cytokine IFN-γ (18) could sensitize intrinsic cells of the vessel wall (ECs and VSMCs) or resident and infiltrating leukocytes of the vessel wall (T cells and macrophages) to the proinflammatory effects of nucleic acids, including self-nucleic acids. We initially studied intact human coronary arteries, confirmed pertinent results and investigated their mechanisms in cultured human cells, and finally verified key findings in a chimeric in vivo model of human coronary artery grafts to immunodeficient mouse recipients. In our study, synthetic analogues were used instead of microbial RNA/DNA as sources of exogenous nucleic acids (9, 19–21) and total RNA from uninfected cells were used as a source of endogenous nucleic acids (22). Specifically, synthetic CpG-containing DNA was used as a substitute for microbial DNA (19), synthetic IFN-stimulatory DNA (ISD) without CpG sequences was used as a substitute for self-DNA (9), the ssRNA analogue, resiquimod (R848) was used as a substitute for microbial ssRNA or self-ssRNA (20), and a synthetic dsRNA, polyinosinic:polycytidylic acid (poly(I:C)) was used as a substitute for microbial dsRNA or self-dsRNA (21). We find that synthetic dsRNA and self-RNA induce cytokine and chemokine production by VSMCs and that IFN-γ enhances these vascular inflammatory responses by upregulation of dsRNA receptor expression.
MATERIALS AND METHODS
Artery and cell culture
Human epicardial coronary arteries were obtained from explanted hearts of transplant recipients and cadaveric organ donors under protocols approved by the institutional review boards of Yale University and the New England Organ Bank. Peri-adventitial fat was removed by careful dissection and the arteries were divided into 3 mm rings. Alternatively, the arteries were finely minced and VSMCs were obtained by explant outgrowth and used after 2–3 passages. The arteries and VSMCs were cultured in M-199 medium supplemented with 20% FBS, 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA). Human ECs were isolated by enzymatic harvesting from umbilical cord veins and serially cultured in supplemented M199 medium also containing 50 μg/mL fibroblast growth factor-1 (Collaborative Research, Bedford, MA). Human PBMCs were obtained by density centrifugation following apheresis of healthy volunteers and cultured in RPMI-1640 medium (Invitrogen) supplemented with 10% FBS. The cells were cultured at 1×103 cells/mm2 for ECs and VSMCs vs. 1×104 cells/mm2 for PBMCs in 24-well plates (BD Falcon, San Jose, CA), except in experiments comparing all three cell types when 96-well plates were used with similar cell densities.
Pharmacologic and biologic agents
Arteries and cells were treated with poly(I:C) (Amersham Biosciences Corp., Piscataway, NJ), R848 (PharmaTech, Shanghai, China), CpG DNA (Invivogen, San Diego, CA), ISD (sense-TAC AGA TCT ACT AGT GAT CTA TGA CTG ATC TGT ACA TGA TCT ACA; antisense-TGT AGA TCA TGT ACA GAT CAG TCA TAG ATC ACT AGT AGA TCT GTA; W.M. Keck Facility, Yale University), LPS (E.Coli 0111:B4; Sigma-Aldrich, St. Louis, MO), JAK inhibitor-1, IKK-2 inhibitor (EMD Chemicals Inc., Gibbstown, NJ), TLR4 antibody (clone HTA125; eBioscience, San Diego, CA), type I IFN receptor chain 2 antibody (clone MMHAR-2; PBL InterferonSource, Piscataway, NJ), or recombinant human IFN-β (PBL InterferonSource). In certain cases, artery and cell cultures were pretreated with recombinant human IFN-γ (Invitrogen) for 3 days. In other cases, cells were treated with nucleic acids mixed at 1:1 wt/vol with lipofectamine (Invitrogen) or 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP; Roche Diagnostics Corp., Indianapolis, IN) in 1 mL serum-free medium for 6 hr prior to adding 20% FBS. For treatment with self-RNA, total RNA (positive strands) was extracted from uninfected VSMC cultures using miRNeasy mini kits (Qiagen, Valencia, CA) which enable purification of RNA greater than approximately 18 nucleotides. In control preparations, the RNA was pretreated with a combination of 10 U/mL RNase H and 10 U/mL RNase A (USB Corp., Cleveland, OH) for 40 min under low salt conditions to allow for digestion of all forms of RNA. The reaction was stopped by repeating the RNA extraction procedure and the RNA was eluted in water.
Transfection of siRNA
Small interfering RNA (siRNA) against TLR3, MDA5 and RIG-I and a non-silencing control sequence without homology to known mammalian genes were obtained from Qiagen. The sequence for TLR3 siRNA was: sense- CGA AUU UGA CUG AAC UCC A dTdT; antisense- UGG AGU UCA GUC AAA UUC G dTdG. The sequence for MDA5 siRNA was: sense- GGU GUA AGA GAG CUA CUA A dTdT; antisense- UUA GUA GCU CUC UUA CAC C dTdG. The sequence for RIG-I siRNA was: sense- GGC GUU CUC UAG AUC CUU U dTdT; antisense- AAA GGA UCU AGA GAA CGC C dTdG. Human VSMCs were pretreated with IFN-γ at 50 ng/ml for 12 hr, then transfected with siRNA at 10–100 nM complexed with oligofectamine in 1 mL serum-free opti-MEM medium (Invitrogen) for 12 hr, and then quenched with serum-containing M199 medium for 12 hr. Fresh supplemented medium was added followed by addition of either poly(I:C) or self-RNA complexed to lipofectamine for 6–48 hr. Cellular RNA and proteins were collected to assess knockdown efficacy and cytokine production.
Real-time RT-PCR
RNA was extracted from cultured VSMCs by RNeasy mini kits (Qiagen) with on-column DNA digestion according to the manufacturer’s protocol. Frozen coronary artery rings after organ culture were prepared for RNA extraction by crushing the specimens on dry ice followed by addition of RLT buffer (Qiagen). Alternatively, RNA was extracted from 20 serial sections (each 20 μm thick) from each coronary artery graft after brief immersion of the sections in water and centrifugation followed by addition of RLT buffer. Reverse transcription was performed using Multiscribe RT reagents (Applied Biosystems, Foster City, CA). Real-time RT-PCR was performed using commercially available Applied Biosystems probes, Taqman Mastermix, and a thermal cycler (iQ5; Bio-Rad Laboratories, Hercules, CA). Samples were analyzed in duplicate and the target gene expression was normalized to that of GAPDH.
ELISA and LAL assay
Cytokine levels were measured using commercial sandwich ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer. The lower limits of detection were 7.8 pg/mL for both IFN-γ and TNF-α, 1.9 pg/mL for IL-1β, 4.6 pg/mL for IL-6, and 15.6 pg/mL for IP-10. Cell culture reagents and nucleic acid preparations were tested for the presence of LPS using a Limulus amebocyte lysate (LAL) assay (Cambrex Bio Science, Walkersville, MD) according to the manufacturer’s instructions. In brief, reagents were added to 50 μl of LAL for 10 min at 37 °C, 100 μl of chromogenic substrate solution were then added for 6 min, and the reaction was stopped with 100 μl of 10% SDS. The concentration of p-nitroaniline was measured at 410 nm using an ELISA plate reader and compared with standard solutions of E. coli endotoxin from 0.3–5 U/mL.
Immunoblotting analysis
Cultured VSMCs were washed with ice-cold phosphate buffered saline and proteins were extracted by adding lysis buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 5 mM EDTA, 1 mM sodium orthovanadate, 0.1% aprotinin, and 1 mM PMSF). The lysate was subjected to ultra-sonification for 15 sec and continuous slow agitation for 24 hr at 4° followed by ultra-centrifugation for 10 min at 4°. The supernatant was boiled in SDS buffer for 5 min at 100°. Equal amounts of protein per sample were separated by SDS-PAGE, transferred electrophoretically to a nitrocellulose membrane (Bio-Rad Laboratories), and immunoblotted with mouse monoclonal antibodies to human IFN-α (Cell Signaling, Boston, MA), TLR3 and β-actin (Abcam, Cambridge, MA), rabbit polyclonal antibody to human MDA5 (ProSci Inc. Poway, CA) or goat polyclonal antibody to human RIG-I (Novus Biologicals, Littleton, CO) followed by relevant horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA). Conjugated antibody was detected by enhanced chemiluminescence (Pierce Biotechnology, Rockford, IL).
Histology and immunohistochemistry
Artery morphology was assessed by elastica–vanGieson staining. Immunohistochemistry was performed using mouse monoclonal antibodies to α-smooth muscle actin (Dako, Carpinteria, CA). Binding of secondary antibody (Jackson Immunoresearch) was detected by peroxidase/3-amino-ethyl carbazole kit (Vector Laboratories, Burlingame, CA). TUNEL labeling was performed using reagents from Chemicon International (Temecula, CA) according to the manufacturer’s protocol.
Coronary artery grafts
Interposition of human coronary artery segments into the infra-renalaortae of female SCID/beige mice (Taconic, Germantown, NY) was performed using an end-to-endmicrosurgical anastomotic technique under protocols approved by the Yale Animal Care and Use Committee. At 1 wk post-operatively, paired recipients of adjacent artery segments were inoculated i.v. with 1 × 1010 optical particle units (OPU) of replication-deficient adenovirus encoding transgenes for LacZ (Ad-LacZ) or human IFN-γ (Ad-IFN-γ) driven by a CMV promoter (Qbiogene, Carlsbad, CA). Circulating human IFN-γ levels were 128 ± 28 ng/mL at 2 wk after Ad-IFN-γ transduction and were undetectable in Ad-LacZ-transduced animals. At 4 wk after viral transduction, certain recipients received poly(I:C) at 150 μg/day i.p. for 3 days. The animals were euthanized at 5 wk post-operatively (4 wk after viral transfection), perfused with normal saline via the heart, and the coronary artery grafts were excised and analyzed.
Statistical analysis
Student’s t test and the Mann-Whitney U test were used to compare two experimental groups with Gaussian and non-Gaussian distributions, respectively. Alternatively, one-way Analysis of Variance and the Kruskal-Wallis analysis of ranks were used to compare more than two experimental groups with Gaussian and non-Gaussian distributions, respectively. P values were two-tailed and values < 0.05 were considered to indicate statistical significance. Statistical analyses were performed using Prism 4 (GraphPad Software, San Diego, CA).
RESULTS
Inflammatory responses of intact human coronary arteries to nucleic acids
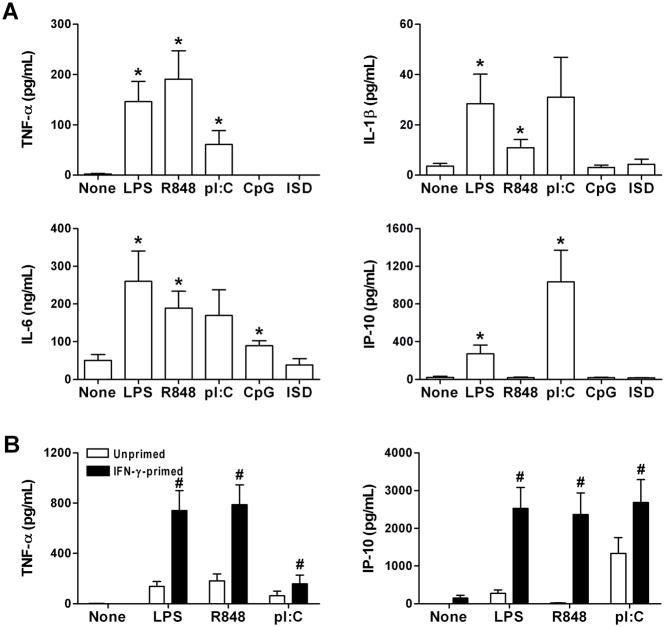
We first examined if nucleic acids may contribute to the pro-inflammatory milieu of arteriosclerosis using an organ culture system. Human coronary artery rings were incubated with various nucleic acids and the levels of secreted cytokines were compared to that of untreated and LPS-treated arteries. The ssRNA analogue, R848 and the synthetic dsRNA, poly(I:C) stimulated secretion of several cytokines (Fig. 1A). In contrast, ssDNA containing microbial CpG motifs or ISD lacking CpG sequences had minimal, if any, pro-inflammatory effects. The stimulatory effects of RNA were similar in arteries with or without gross atherosclerotic disease and the data were pooled for greater statistical power. TNF-α secretion was greatest in response to R848, whereas IP-10 secretion was most robust after poly(I:C) treatment. The supernatant levels of IL-1β from treated arteries were relatively low, while there was high basal secretion of IL-6 from untreated arteries. We therefore focused our further studies on the effects of RNA on the production of TNF-α and IP-10.
Figure 1.
RNA induces cytokine production by human coronary arteries in organ culture and this response is augmented by IFN-γ priming. (A) Artery rings were treated with medium alone (None) or LPS, R848, poly(I:C) (pI:C), CpG-containing ssDNA, and ISD dsDNA without CpG sequences at 1 μg/mL for 48 hr. TNF-α, IL-1β, IL-6, and IP-10 supernatant levels were measured by ELISA. Data are means ± SE (n = 4–18 donors with and without atherosclerotic disease). *P < 0.05 Treated vs. None. (B) Artery rings were pretreated or not with IFN-γ at 40 ng/mL for 72 hr prior to treatment with nucleic acids. Data are means ± SE (n = 13–15 donors with and without atherosclerotic disease). #P < 0.05 IFN-γ-primed vs. Unprimed.
IFN-γ potentiates the inflammatory response of human coronary arteries to RNA
We next tested if IFN-γ, a cytokine linked to arteriosclerosis (18), modulated arterial inflammatory responses to nucleic acids. Coronary artery rings were pretreated with IFN-γ for 3 days, washed, and then treated with R848 or poly(I:C) vs. LPS or no treatment. IFN-γ alone did not induce TNF-α secretion and after 3 days there was little residual production of IFN-γ-inducible IP-10 (pilot experiments had established that IFN-γ activity in the supernatant progressively declined over 2 days following recombinant IFN-γ administration and was absent after 3 days, as measured by STAT1 phosphorylation of naïve cultures treated with conditioned medium). Strikingly, IFN-γ pretreatment of arteries significantly enhanced the subsequent production of TNF-α and IP-10 in response to R848 or poly(I:C) administration (Fig. 1B). These results established that RNA, but not DNA, elicited detectable inflammatory responses by human coronary arteries and that this effect was greatly augmented by IFN-γ priming.
dsRNA induces TNF-α and IP-10 production in IFN-γ-primed, cultured human VSMCs
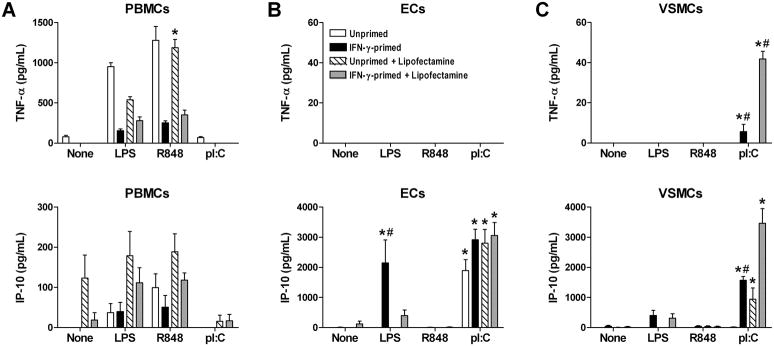
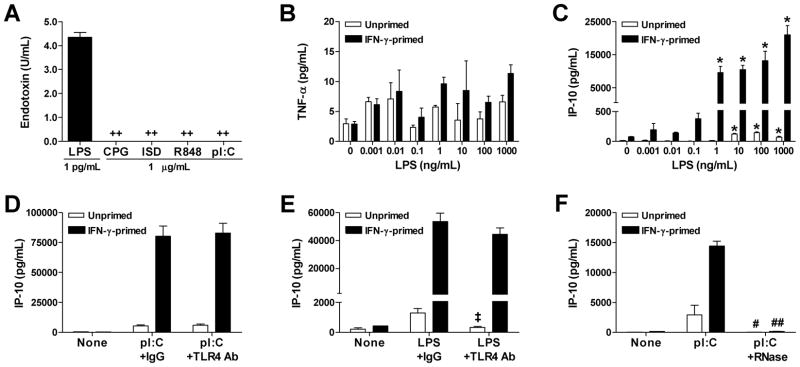
Since human coronary arteries contain several cell types, including resident or infiltrating leukocytes even in the absence of arteriosclerotic disease (23, 24), we dissected the contributions of various cell types to the pro-inflammatory responses of arteries to RNA using purified populations of cultured vascular cells and circulating leukocytes. Discordantly, ssRNA, but not dsRNA, stimulated TNF-α and IP-10 production by PBMCs (Fig. 2A). Conversely, dsRNA, but not ssRNA, induced TNF-α production by VSMCs and IP-10 production by ECs and VSMCs (Fig. 2B, C). There were no synergistic effects of combined ssRNA and dsRNA treatment on any of the cell types (data not shown). ECs did not produce TNF-α under any condition and their production of IP-10 in response to poly(I:C) was not modulated by IFN-γ pretreatment. On the other hand, VSMCs secreted relatively small amounts of TNF-α (about 20-fold less than maximal PBMC responses) and relatively large amounts of IP-10 (about 20-fold more than maximal PBMC responses) in response to poly(I:C). TNF production by VSMC was dependent on IFN-γ priming and IP-10 production by VSMC was greatly augmented (10-fold) by IFN-γ priming, particularly when RNA was complexed to a transfection agent in order to enhance cytosolic access. Dose response experiments with poly(I:C) confirmed that lipofectamine and other cationic lipids, such as DOTAP, augmented dsRNA-induced cytokine production by VSMCs (Table I). The effects of RNA in our systems were unlikely to be due to contaminating microbial products as endotoxin levels were <0.1 U/mL in the nucleic acid preparations and were less than that in 1 pg/mL LPS (Fig. 3A), far higher concentrations of 1 and 10 ng/mL of LPS were required to elicit IP-10 production by unprimed and IFN-γ-primed VSMCs, respectively (Fig. 3B), even higher doses of LPS were not effective in inducing TNF-α secretion by VSMC (Fig. 3C), a neutralizing antibody to the LPS receptor, TLR4 did not affect IP-10 production in response to poly(I:C), but did inhibit LPS responses of unprimed VSMCs (Fig. 3D, E), and the biologic effects of poly(I:C) on unprimed and IFN-γ-primed VSMCs was abolished by prior RNase digestion (Fig. 3F). Our data on isolated cell responses to RNA indicated that the most profound effects of IFN-γ priming were on VSMCs, the major cellular constituent of the vessel wall, and we focused our further studies on this cell type.
Figure 2.
IFN-γ primes cultured VSMCs for inflammatory responses to dsRNA. (A) PBMCs, (B) ECs, and (C) VSMCs were primed or not with IFN-γ at 40 ng/mL for 72 hr followed by treatment with medium alone (None), LPS, R848, or poly(I:C) at 1 μg/mL, delivered with or without lipofectamine, for 48 hr. TNF-α and IP-10 supernatant levels were measured by ELISA. Data are means ± SE (n = 4 for PBMCs and n = 8 for ECs and VSMCs). *P < 0.05 Treated vs. None and #P < 0.05 IFN-γ-primed vs. Unprimed.
Table I.
Cationic lipids enhance cytokine production by VSMCs in response to dsRNAa
| pI:C Dose | TNF-α (pg/mL) |
IP-10 (ng/mL) |
||||
|---|---|---|---|---|---|---|
| None | Lipofectamine | DOTAP | None | Lipofectamine | DOTAP | |
| 0 μg/mL | 3.8±0.1 | 3.9±0.2 | 4.7±0.2 | 0.1±0.0 | 0.5±0.0 | 0.9±0.1 |
| 0.1 μg/mL | 4.0±0.1 | 4.9±0.1 | 4.3±0.1 | 2.0±0.2 | 10.6±0.5 | 10.9±0.6 |
| 1 μg/mL | 8.1±3.4 | 35.9±5.5*# | 68.5±15.6*# | 6.5±0.2 | 34.5±2.7*# | 23.2±6.5*# |
| 10 μg/mL | 15.5±4.1 | 63.2±1.6*# | 86.2±5.8*# | 7.1±0.2 | 28.4±6.3*# | 24.8±0.6*# |
VSMCs were treated with poly(I:C) (pI:C) at various doses delivered alone (None) or with lipofectamine or DOTAP, the supernatant was collected after 48 hr, and TNF-α and IP-10 levels were measured by ELISA. Note that concentrations are 1,000-fold greater for IP-10 than for TNF-α, consistent with differences in receptor affinities for these ligands. Data are means ± SE (n = 4).
P < 0.01 vs. Poly(I:C) at 0 μg/mL and
P < 0.01 vs. No Cationic lipid (None).
Figure 3.
Microbial contaminants do not contribute to dsRNA-induced VSMC inflammatory responses. (A) Endotoxin levels were measured by LAL in preparations of nucleic acids at 1 μg/mL and compared to that of LPS at 1 pg/mL. Data are means ± SE (n = 3). ++P < 0.001 Nucleic acids vs. LPS. (B) TNF-α and (C) IP-10 supernatant levels from unprimed and IFN-γ-primed VSMCs treated with various doses of LPS for 48 hr. Data are means ± SE (n = 3). *P < 0.05 LPS-treated vs. Untreated. Additionally, IP-10 supernatant levels were measured from VSMCs treated with (D) poly(I:C) at 1 μg/mL or (E) LPS at 10 ng/mL for 48 hr in the presence of neutralizing antibody to TLR4 or isotype-matched, irrelevant IgG at 10 μg/mL. Data are means ± SE (n = 4–8). ‡P < 0.05 TLR4 Ab vs. IgG. (F) IP-10 secretion from VSMCs treated with poly(I:C) or RNase-digested poly(I:C) for 48 hr. Data are means ± SE (n = 4). #P < 0.05 and ##P < 0.001 pI:C vs. pI:C + RNase. Unprimed and IFN-γ-primed VSMCs were compared separately in panels B–F.
dsRNA induces type I IFN production and responses in coronary arteries and VSMCs
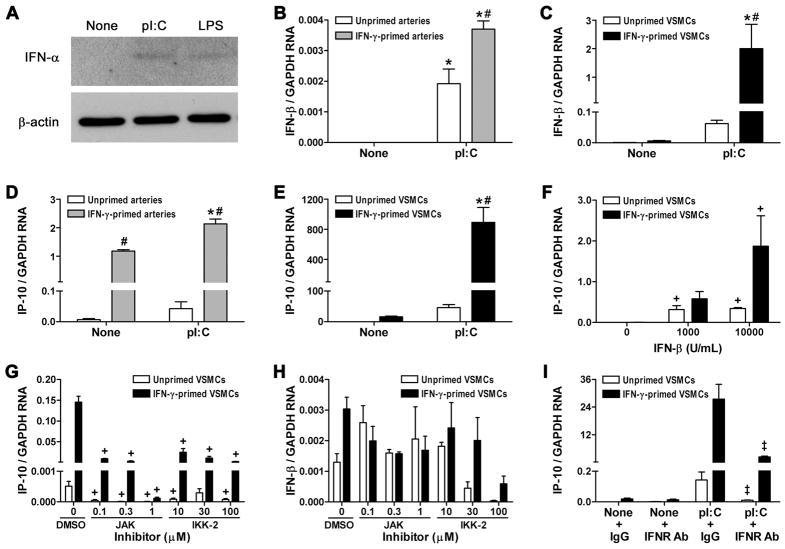
Type I IFNs are a major component of inflammatory responses to nucleic acids via IRF3 and IRF7 activation (7). We therefore attempted to measure levels of IFN-α and IFN-β in the supernatants of treated coronary arteries and VSMCs, but were unsuccessful as the commercial kits then available had relatively high minimum detection levels at or above the secreted cytokine concentrations. Nonetheless, immunoblotting confirmed induction of type I IFN protein by poly(I:C)-treated VSMCs (Fig. 4A). As an alternative quantitative technique, we measured the expression of type I IFN transcripts by real-time RT-PCR. IFN-β was undetectable in untreated or IFN-γ-primed human coronary arteries, but was markedly induced by poly(I:C) treatment (Fig. 4B). Similar results were obtained in cultured human VSMCs with robust effects of IFN-γ pretreatment (Fig. 4C). Thus, IFN-β transcript analysis represented an additional readout for the pro-inflammatory effects of dsRNA on VSMCs.
Figure 4.
Type I IFN production and responses by dsRNA-treated arteries and VSMCs. (A) Immunoblotting for IFN-α and β-actin in lysates of VSMCs treated with poly(I:C) or LPS at 1 μg/mL for 48 hr. (B) Coronary artery rings and (C) VSMCs, primed or not with IFN-γ at 50 ng/mL for 72 hr, were not treated (None) or treated with poly(I:C) at 1 μg/mL for 48 hr, and IFN-β transcript levels normalized to GAPDH were measured by real-time RT-PCR. IP-10 transcripts were also measured from the (D) coronary arteries and (E) VSMCs. Data are mean ± SE (n = 2–3). *P < 0.05 Poly(I:C)-treated vs. None and #P < 0.05 IFN-γ-primed vs. Unprimed. (F) IP-10 transcripts in VSMCs treated with IFN-β at various concentrations for 48 hr. Data are mean ± SE (n = 4). +P < 0.05 IFN-β-treated vs. Untreated. Alternatively, VSMCs were treated with poly(I:C) at 1 μg/mL for 48 hr in the presence of DMSO vehicle or JAK or IKK-2 inhibitors at various concentrations and (G) IP-10 and (H) IFN-β transcripts were determined. Data are mean ± SE (n = 3). +P < 0.05 Inhibitor vs. DMSO. (I) IP-10 transcripts in VSMCs treated or not with poly(I:C) at 1 μg/mL for 48 hr in the presence of neutralizing antibody to the type I IFN receptor (IFNR) or isotype-matched, irrelevant IgG at 10 μg/mL. Data are mean ± SE (n = 3). ‡P < 0.05 IFNR Ab vs. IgG. Unprimed and IFN-γ-primed VSMCs were compared separately in panels F–I.
Since IFN-β may directly induce IP-10 secretion by VSMCs (23), we investigated whether the production of IP-10 in our system was indirectly dependent on type I IFN-induced JAK/STAT activation and/or directly dependent on NFκB activation, two major signaling pathways in dsRNA-induced inflammatory responses (7). We confirmed that poly(I:C) increased IP-10 transcripts in addition to that of IFN-β in cultured arteries and VSMCs (Fig. 4D, E) and that IFN-β also induced accumulation of IP-10 transcripts in VSMCs (Fig. 4F). Both a JAK inhibitor with inhibitory activity to JAK1 and Tyk2 as well as an IKK-2 inhibitor which blocks NFκB-dependent gene expression greatly decreased IP-10 secretion by poly(I:C)-treated VSMCs (Fig. 4G). The inhibitory effects were unlikely to be due to reagent toxicity and cell death as poly(I:C)-induced, IRF3/IRF7-dependent IFN-β transcripts were not significantly modulated by the same treatments (Fig. 4H) and supernatant levels of intracellular components, such as LDH were not increased (data not shown). As pharmacologic agents may have non-specific effects, we verified that IFN-β partially contributed to IP-10 production in poly(I:C)-treated VSMCs using blocking antibodies to the type I IFN receptor (Fig. 4I). Thus, IP-10 was a robust indicator of dsRNA effects on VSMC due to both direct activation of NFκB signaling as well as indirect JAK/STAT signaling via type I IFN production.
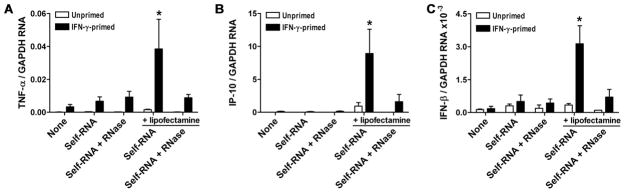
Self-RNA induces inflammatory responses in IFN-γ-primed VSMCs
So far a substitute for exogenous dsRNA, poly(I:C) was investigated. To test for possible responses to endogenous sources of nucleic acids, such as that released from dying cells, we also extracted total RNA of >18 nucleotide lengths from uninfected VSMCs, referred to as self-RNA. The self-RNA preparations had undetectable endotoxin levels. Treatment with self-RNA did not induce accumulation of cytokine transcripts in unprimed or IFN-γ-primed VSMCs, except when complexed with lipofectamine (Fig. 5A–C). The specificity of these limited responses was confirmed by RNase pretreatment of the self-RNA preparations with consequent loss of immunostimulatory activity. The production of pro-inflammatory cytokines in response to self-RNA delivered with lipofectamine was not diminished in the presence of blocking antibodies to TLR4, excluding biologically relevant levels of LPS contamination in our preparations (data not shown). These results demonstrated that under certain circumstances, such as exosome fusion which lipofectamine-mediated uptake of nucleic acids mimics, VSMCs may also respond to endogenous RNA that are known to accumulate in arteriosclerotic lesions.
Figure 5.
Self-RNA induces inflammatory responses in IFN-γ-primed VSMCs. VSMCs were either primed or not with IFN-γ at 100 ng/mL for 72 hr followed by treatment with self-RNA at 1 μg/ml, with or without lipofectamine, for 18 hr. Control self-RNA preparations were pretreated with RNases. Cellular RNA was extracted and transcript levels of (A) TNF-α, (B) IP-10, and (C) IFN-β were measured by real-time RT-PCR and normalized to GAPDH mRNA. Data are means ± SE (n = 4–8) and the results were pooled from 4 experiments. *P < 0.05 Treated vs. None.
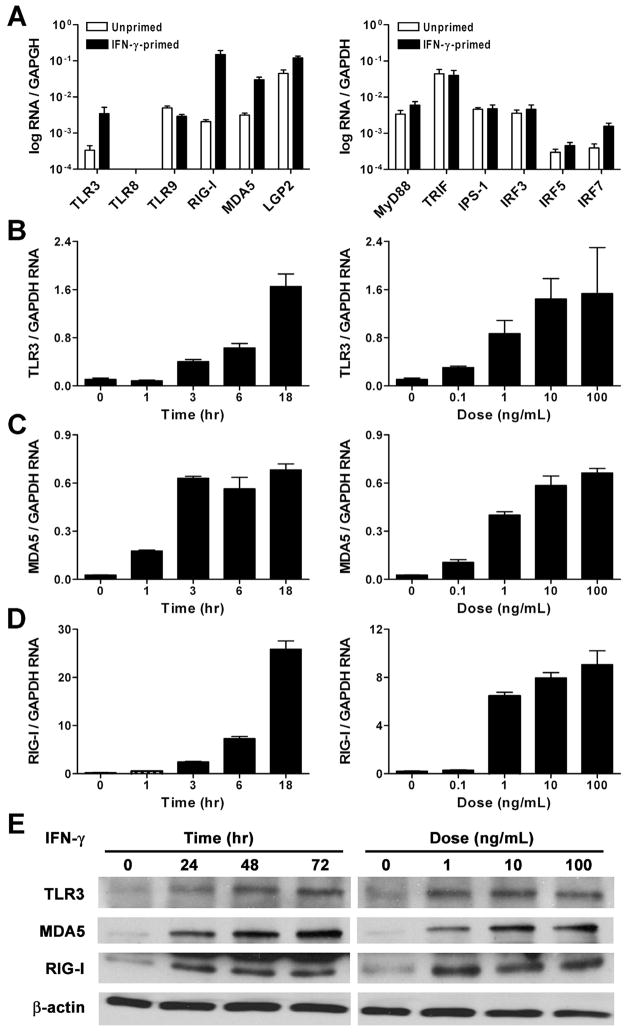
IFN-γ upregulates the expression of dsRNA receptors
We next examined if the VSMC priming effect was due to IFN-γ-mediated regulation of molecules involved in innate recognition of nucleic acids. Human coronary arteries without gross atherosclerosis were treated with IFN-γ and the expression of several PRRs for nucleic acids was determined by quantitative real-time RT-PCR. Transcripts for the dsRNA receptors, TLR3, MDA5, and RIG-I were increased by 10- to 100-fold, whereas the ssRNA receptor, TLR8, the DNA receptor, TLR9, and the non-signaling dsRNA receptor, laboratory of genetics and physiology-2 were undetectable or minimally altered (Fig. 6A). Of numerous adaptor and signaling molecules involved in inflammatory responses to nucleic acids, only the expression of IRF7 transcripts was modestly upregulated 4-fold. Detailed time and dose response experiments in cultured VSMCs confirmed a 15-fold increase in TLR3, a 25-fold increase in MDA5, and up to a 120-fold increase in RIG-I transcripts (Fig. 6B–D). IFN-γ-mediated induction of TLR3, MDA5, and RIG-I protein expression in VSMCs was verified by immunoblotting (Fig. 6E). The regulation of dsRNA receptors by IFN-γ provided a possible mechanism for the IFN-γ-mediated priming of VSMCs to pro-inflammatory effects of dsRNA.
Figure 6.
IFN-γ upregulates the expression of dsRNA receptors in arteries and VSMCs. (A) Non-atherosclerotic coronary artery segments were either treated or not with IFN-γ at 40 ng/mL for 72 hr, RNA was extracted, and transcript levels of nucleic acid receptors as well as their adaptor and signaling molecules were determined by real-time RT-PCR and normalized to GAPDH mRNA. Cultured VSMCs were treated with IFN-γ at 100 ng/mL for various times or at different doses for 18 hr following which (B) TLR3, (C) MDA5, and (D) RIG-I transcript levels were measured. (E) Additionally, VSMCs were treated with IFN-γ at 100 ng/mL for various times or at different doses for 72 hr and cell lysates were immunoblotted for TLR3, MDA5, RIG-I, and β-actin. Quantitative data are means ± SE (n = 3). Immunoblots are representative of 3 experiments.
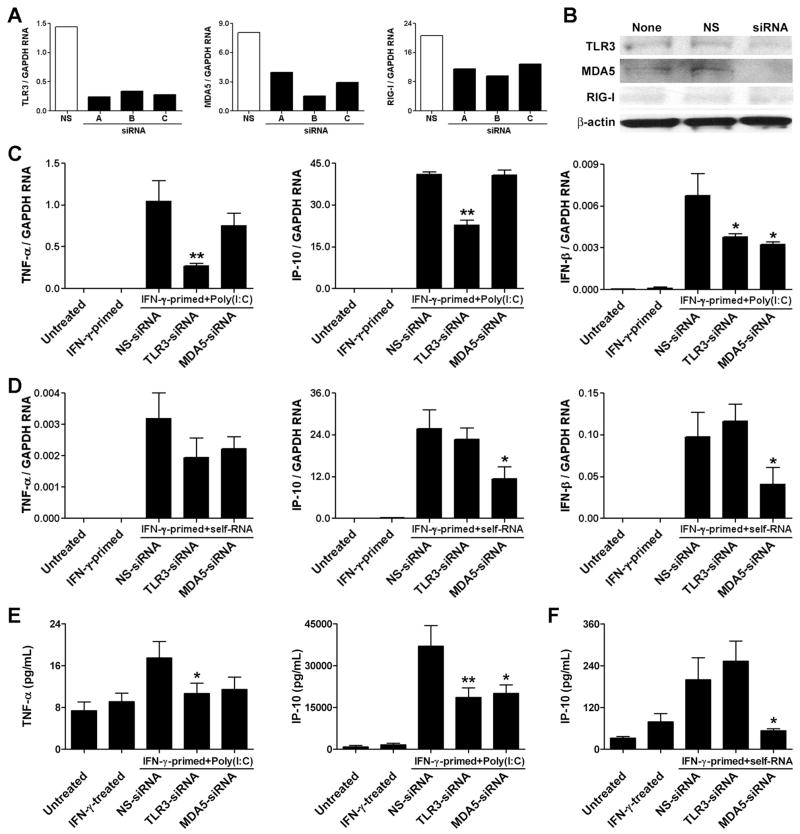
TLR3 and MDA5 are necessary for IFN-γ-primed VSMC responses to dsRNA
We investigated which IFN-γ-inducible dsRNA receptors are required for the inflammatory responses of cultured VSMCs to nucleic acids. The expression of TLR3 and MDA5 transcripts in IFN-γ-treated VSMCs was inhibited by >80% using optimal siRNA sequences (Fig. 7A). Under the same conditions, the expression of RIG-I RNA could not be diminished by more than 50% using three different siRNA sequences. Immunoblotting confirmed the knockdown of TLR3 and MDA5, but not of RIG-I, protein expression in IFN-γ-treated VSMCs (Fig. 7B). We therefore did not further consider the role of RIG-I in our system. Importantly, we excluded a direct pro-inflammatory effect of the siRNA sequences on unprimed and IFN-γ-primed VSMCs. Compared to untreated controls, IP-10 transcript accumulation was 0.7, 1.2, and 1.0-fold in response to non-stimulating, TLR3, and MDA5 siRNA at 50 nM, respectively vs. 9,485-fold higher in response to poly(I:C) at 1 μg/mL. TLR3 knockdown significantly diminished the production of TNF-α, IP-10, and IFN-β by IFN-γ-primed VSMCs in response to poly(I:C) treatment (Fig. 7C). MDA5 knockdown was less effective in preventing the inflammatory response to synthetic dsRNA and only reduced IFN-β transcript accumulation. In contrast, MDA5, but not TLR3, knockdown effectively decreased cytokine production by IFN-γ-primed VSMCs in response to self-RNA complexed to lipofectamine (Fig. 7D). Similar results were obtained with alternative siRNA sequences (data not shown). ELISA analyses of cells from different donors confirmed the real-time PCR results (Fig. 7E, F), although TNF-α secretion by VSMCs was below detectable limits after self-RNA stimulation (data not shown). The siRNA knockdown results demonstrated that both TLR3 and MDA5 contributed to IFN-γ priming of VSMC inflammatory responses to RNA, depending on the source of the nucleic acids.
Figure 7.
dsRNA receptors contribute to cytokine expression in poly(I:C)-stimulated, IFN-γ-primed VSMC. (A) VSMCs were transfected with either control non-silencing (NS) siRNA or 3 different siRNA sequences to TLR3, MDA5, and RIG-I for 24 hr and then treated with IFN-γ at 10 ng/mL for 72 hr. RNA was extracted and TLR3, MDA5, and RIG-I transcript levels were determined by real-time RT-PCR and normalized to GAPDH mRNA. (B) Additionally, lysates were extracted from siRNA-transfected, IFN-γ-primed VSMCs and immunobloted for TLR3, MDA5, RIG-I, and β-actin. (C) IFN-γ-primed VSMCs were transfected with either NS-siRNA, TLR3 siRNA, or MDA5 siRNA for 24 hr and then treated with poly(I:C) at 1 μg/mL for 6 hr. RNA was extracted and TNF-α, IP-10, and IFN-β transcript levels were measured. (D) Alternatively, siRNA-transfected, IFN-γ-primed VSMCs were treated with self-RNA at 1 μg/mL complexed with lipofectamine for 6 hr, and TNF-α, IP-10, and IFN-β transcript levels were measured. Data are means ± SE (n = 3) and are representative of 5 experiments with poly(I:C) and 2 experiments with self-RNA. *P < 0.05 and **P < 0.01 siRNA vs. NS-siRNA. Additionally, the siRNA effects were confirmed by ELISA analysis of secreted TNF-α and IP-10 from IFN-γ-primed VSMC treated with poly(I:C) for 48 hr (E) and of secreted IP-10 in response to self-RNA complexed to lipofectamine for 48 hr (F). Data are means ± SE (n = 12 from 2 donors). *P < 0.05 and **P < 0.01 siRNA vs. NS-siRNA.
IFN-γ primes human coronary arteries to dsRNA-mediated inflammatory responses in vivo
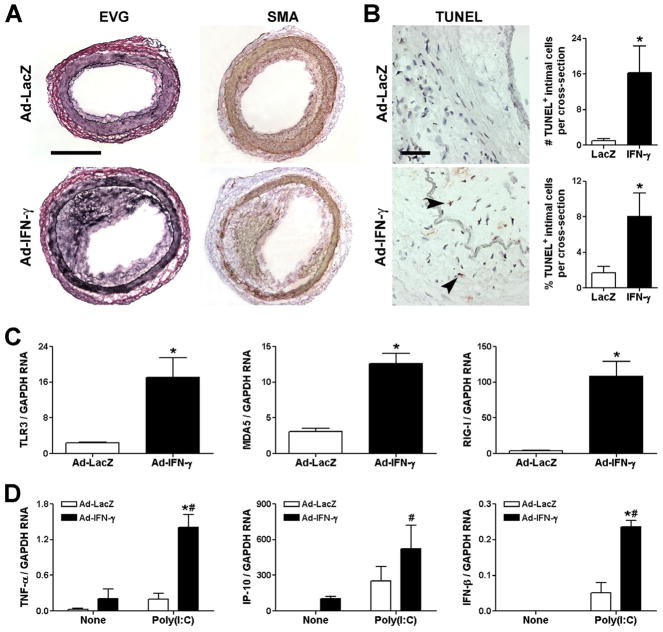
Finally, we examined if our in vitro observations were of relevance in vivo. Human coronary artery segments were interposed into the aortae of immunodeficient mice and the recipients were subsequently inoculated with adenovirus encoding human IFN-γ or the control transgene, LacZ. We confirmed our previous observations (24, 25) that exposure of the grafts to circulating IFN-γ for 4 wk resulted in VSMC hyperplasia, intimal expansion, and outward vascular remodeling (Fig. 8A). There was also evidence of IFN-γ resulting in VSMC death by increased detection of TUNEL+ apoptotic cells, particularly within the neointima (Fig. 8B), suggesting a possible endogenous source for nucleic acids. Chronic exposure to IFN-γ greatly increased graft expression of TLR3, MDA5, and RIG-I transcripts (Fig. 8C). Additionally, TNF-α and IP-10, but not IFN-β, transcripts were detectable by real-time RT-PCR in IFN-γ-treated arteries (Fig. 8D). Administration of poly(I:C) shortly before harvest of the grafts increased the production of TNF-α, IP-10, and IFN-β and this effect was significantly augmented in IFN-γ-treated hosts. The cells contributing to the IFN-γ-induced intimal expansion in this model are known to be VSMC of human origin (24, 25), and the TNF-α, IP-10, and IFN-β detected within the grafts are produced by human cells as the species-specific PCR probes do not recognize mouse cytokine and chemokine transcripts (data not shown). These results confirmed that the IFN-γ-mediated regulation of dsRNA receptors and the IFN-γ priming of dsRNA-induced inflammatory responses seen in vitro also occurred in vivo.
Figure 8.
IFN-γ induces vascular expression of dsRNA receptors and primes arteries for inflammatory responses to dsRNA in vivo. Human coronary artery segments were interposed into the infra-renal aorta of SCID/beige mice, adenovirus (Ad) encoding LacZ or human IFN-γ was inoculated i.v. at 1010 OPU after 1 wk, and the human vessel grafts were removed for analysis after 5 wk (i.e. 4 wk after viral infection). (A) Morphology was examined by elastin-van Gieson (EVG) staining and α-smooth muscle actin (SMA) immunostaining, bar represents 400 μm. (B) Apoptotic cells were detected by TUNEL staining (arrows indicate TUNEL+ nuclei), lumen orientated to upper right, bar represents 50 μm, and the frequency of intimal TUNEL+ cells was calculated as absolute numbers and as % of total cells. Data are means ± SE (n = 7 animals per group). *P < 0.05 Ad-IFN-γ vs. Ad-LacZ. (C) TLR3, MDA5, and RIG-I transcript levels in human artery grafts from Ad-LacZ or Ad-IFN-γ inoculated hosts were determined by real-time RT-PCR and normalized to GAPDH. Data are means ± SE (n = 4 animals per group). *P < 0.05 Ad-IFN-γ vs. Ad-LacZ. (D) Additionally, at 4 wk after Ad-LacZ or Ad-IFN-γ inoculation certain hosts were treated with poly(I:C) at 150 μg i.p. per day for 3 days prior to artery graft excision and RNA extraction, whereas matched control recipients, labeled “None”, did not receive poly(I:C). Transcript levels of TNF-α, IP-10, and IFN-β were measured. Data are means ± SE (n = 4 animals per group). *P < 0.05 Ad-IFN-γ vs. Ad-LacZ and #P < 0.05 Poly(I:C) vs. None.
DISCUSSION
Our work investigates the pro-inflammatory effects of exogenous model nucleic acids and endogenous nucleic acids on human arteries and the different vascular cell types that are present within the vessel wall. We used model nucleic acids because these agents are known to work through specific PRRs. We also tested the pro-inflammatory effects of total RNA extracted from autologous cultured vascular cells. The latter may differ from degraded or oxidized nucleic acids that accumulate within arteriosclerotic lesions, a potential limitation of these studies. We find: 1) that exogenous RNA, but not DNA, results in robust inflammatory responses by human coronary arteries; 2) that dsRNA, but not ssRNA, elicits cytokine and chemokine production by intrinsic vascular cells, and conversely ssRNA, but not dsRNA, activates artery-infiltrating leukocytes; 3) that self-RNA, in addition to synthetic dsRNA, stimulate VSMCs; and most significantly 4) that IFN-γ sensitizes VSMCs to RNA-induced pro-inflammatory effects by upregulation of TLR3 and MDA5 expression.
Previous studies have found that synthetic dsRNA induces inflammatory responses in cultured human smooth muscle cells via TLR3 (17, 26). In one of these studies, cultured VSMCs were found to be extremely sensitive to poly(I:C) due to the constitutive expression of TLR3 and siRNA knockdown of TLR3 argued against non-redundant roles for other dsRNA receptors (17). In contrast, we find that untreated VSMCs are only modestly responsive to customary concentrations of poly(I:C) because of relatively low levels of dsRNA receptor expression. These differences may be related to differences in human donors, in culture conditions, and in treatment conditions. We can sensitize cultured VSMCs to poly(I:C) by pretreatment of the cells with IFN-γ. Our findings with cultured cells are consistent with our analysis of intact arteries in organ culture and after transplantation into immunodeficient mice. We further extend previous findings with cultured cells by determining the effects of self-RNA and IFN-γ, and by investigating the role of cytoplasmic dsRNA receptors. As first reported by Yang et al. (17), our experiments with poly(I:C) support a primary role of TLR3 for recognition of this particular dsRNA ligand in cultured VSMCs and we also confirmed their finding that poly(I:C) induces IL-6 secretion from untreated and IFN-γ-primed VSMCs (unpublished observations). A novel observation is that the expression of MDA5 is more important for responses to self-RNA. The concept of various dsRNA receptors playing selective roles in response to different lengths of synthetic dsRNA and to different types of dsRNA viruses is well established (7, 8). Unfortunately, satisfactory knockdown of IFN-γ-induced RIG-I could not be achieved in our system, although RIG-I sensing of dsRNA may inhibit inflammatory responses in certain cell types (27). Our studies were not an exhaustive analysis of dsRNA sensors, such as protein kinase R and the cryopyrin/Nalp3 inflammasome (28, 29). We have also not excluded that generation of small duplex self-RNA by RNase L activated by IFN-inducible 2′,5′-linked oligodenylate contributed to the inflammatory responses in our cells (30). Although we verified the robust induction of dsRNA receptors by IFN-γ seen in intact coronary arteries in cultured VSMCs as well, we did not further assess VSMC expression of other nucleic acid receptors and their associated signaling molecules that were not modulated by cytokine treatment in cultured arteries. However, our previous microarray analysis of IFN-γ-treated VSMCs similarly showed only significant induction of TLR3 (2.3-fold), MDA5 (8.3-fold), RIG-I (13.0-fold), and IRF-7 (4.9-fold) transcripts in cultured cells, but <2-fold changes in that of other molecules involved in detecting innate immune stimuli (31).
We were unable to detect robust cytokine production by human coronary arteries in response to DNA, and in comparison the pro-inflammatory effects of RNA were more compelling. The reasons for the disparate magnitude of inflammatory responses to DNA vs. RNA are not known, but may be related to differing uptake of nucleic acids by specific cell types. For example genomic DNA, but not total RNA, is known to activate rat thyroid cells (32). Synthetic dsDNA and genomic DNA delivered using lipofectamine did induce modest inflammatory responses in our cultured VSMCs (unpublished data). Both the recently described cytosolic dsDNA receptors, DNA-dependent activator of IFN-regulatory factors (33) and absent in melanoma 2 (34) are induced by IFN-γ in human coronary arteries and VSMCs (unpublished data). Our observation that vascular cells respond predominantly to dsRNA and that leukocytes respond predominantly to ssRNA is in agreement with previous reports that human T cells, dendritic cells, and macrophages have limited or absent signaling responses and cytokine production in response to dsRNA, unlike human ECs and fibroblasts or mouse phagocytes (35, 36). The cell type- and species-specific responses to poly(I:C) is not explained by differences in TLR3 expression and likely differences in signaling intermediaries have not been elucidated. These observations suggest that the clinical associations between arteriosclerosis and virus infections which generate dsRNA may reflect inflammatory responses of intrinsic vascular cells rather than artery-infiltrating leukocytes. However, VSMC may be activated by a broader range of viruses than suggested by our results, as DNA and positive-strand ssRNA viruses may generate dsRNA intermediary products in the cytoplasm of infected host cells (37).
We observe a significant priming effect of IFN-γ for inflammatory responses to dsRNA in VSMCs, an example of adaptive immunity instructing non-immune cells in PRR-initiated innate immunity. IFN-γ is well characterized as focusing and enhancing immune responses by macrophages and dendritic cells as well as increasing the antigenicity and pro-inflammatory responses of parenchymal cells and vascular cells (18). Our new work shows that the IFN-γ-dependent sensitization of VSMCs to activation by RNA is mediated by upregulation of dsRNA receptor expression. Previous studies have shown that IFN-γ can prime a human macrophage line for activation by ssRNA via upregulation of TLR7 and TLR8 expression (38) and that type I IFN primes human keratinocytes for activation by poly(I:C) which was associated with increased dsRNA receptor expression (39). In our study, IFN-γ pretreatment or transfection with lipofectamine did not enhance EC activation by dsRNA, perhaps due to the constitutive surface expression of TLR3 on this cell type (36). We confirmed the priming effect of IFN-γ on dsRNA-mediated activation of human coronary arteries in vivo using immunodeficient mouse hosts. The species restriction of IFN-γ activity ensured cytokine effects only on the human vascular graft. Human coronary arteries had minimal responses to poly(I:C) in the absence of IFN-γ, manifesting as modest accumulation of transcripts for TNF-α and IP-10 and undetectable transcript levels for IFN-β. In contrast, there was significant production of all three pro-inflammatory factors in response to poly(I:C) after IFN-γ exposure. However, due to technical challenges in knocking down the expression of intracellular molecules in our chimeric human artery-mouse model (31), we did not perform mechanistic experiments to assess the role of individual dsRNA receptors whose expression had been greatly induced by IFN-γ. Our in vivo data verifies the in vitro observations that adaptive immunity sensitizes tissues to the effects of PAMPs or DAMPs, a corollary to the well-known findings that PAMPs or DAMPs may influence adaptive immune responses.
Another important finding of our study is that self-RNA can activate VSMCs under certain conditions. Previous studies have shown that RNA from necrotic autologous cells can activate TLR3 and induce inflammatory responses in human and murine cells (10, 13). We add to these findings by demonstrating a role for MDA5 in recognizing self-RNA. The inflammatory response to self-RNA was dependent on both IFN-γ pretreatment of VSMCs and transfection using lipofectamine. Lipofectamine-mediated uptake of nucleic acids may mimic some endogenous pathways, such as exosome fusion. Total RNA was used in our studies to simulate endogenous RNA that may be released from tissue damage, similar to the approach used in previous studies (22, 32). A weakness of our methodology is the inability to quantify levels of dsRNA in self-RNA, such as endogenous siRNA or short duplexes arising from the secondary structure of ssRNA. The presence of dsRNA in our self-RNA preparations is inferred from the inflammatory responses of VSMCs which are not activated by ssRNA and by the dependence of this response on dsRNA receptor expression. In addition to the shorter length of duplexes, the likely lower levels of dsRNA in our total RNA extracts from autologous cells than in the synthetic reagents may also explain the weaker inflammatory effect of self-RNA as compared to equivalent concentrations of poly(I:C). Endogenous RNA may be released during cellular turnover, stress, or injury, such as has been described for other DAMPs or “alarmins” (40). Interestingly, the growing alarmin family derived from injured cells includes ribonucleases (41). Alternatively, self-RNA may be both processed and detected within healthy cells via the IFN-dependent activation of RNase L (30). Possible sources of endogenous dsRNA which may instigate inflammation in arteriosclerosis remain speculative at present and range from circulating RNA that is particle-associated (42) to the local accumulation of degraded and oxidized RNA in atherosclerotic plaques (15). In support of the hypothesis that dying cells within the vessel wall are a source of endogenous dsRNA that elicit inflammation, VSMCs undergoing apoptosis initiate an inflammatory program (43), phagocytosis of apoptotic VSMCs by other healthy VSMCs induce the production of inflammatory chemokines (44), and apoptotic VSMCs are associated with inflammation in clinical specimens and experimental models of atherosclerosis (45, 46). Similarly, we find evidence of both VSMC apoptosis and inflammatory responses in our in vivo model. However, there are multiple other candidate alarmins that may be released from dying vascular cells besides nucleic acids, including IL-1α (47–49). Notably, in a system of induced apoptosis in vivo, viable VSMC secrete cytokines and chemokines in response to sensing death of neighboring cells (49).
In conclusion, human coronary arteries and VSMCs respond to exogenous dsRNA and endogenous RNA by production of pro-inflammatory mediators. In the setting of atherosclerosis or graft arteriosclerosis this response may be augmented by IFN-γ through upregulation of dsRNA receptors that may lead to progression of the disease process.
Nonstandard abbreviations
- DAMPs
damage-associated molecular patterns
- DOTAP
1,2-dioleoyl-3-trimethylammonium-propane
- ECs
endothelial cells
- IPS-1
IFN-β promoter stimulator-1
- IP-10
interferon-γ-induced protein of 10 kDa
- IRF
interferon regulatory factor
- ISD
interferon stimulatory DNA
- MDA5
melanoma differentiation-associated gene 5
- PAMPs
pathogen-associated molecular patterns
- PRR
pathogen recognition receptor
- poly(I:C))
polyinosinic:polycytidylic acid
- R848
resiquimod
- RIG-I
retinoic acid-inducible gene I
- siRNA
small interfering RNA
- TRIF
TIR domain-containing adaptor inducing interferon-β
- VSMCs
vascular smooth muscle cells
Footnotes
DISCLOSURES
The authors have no financial conflict of interest.
This work was supported by the NIH (PO1 HL70295).
References
- 1.Hansson GK, Libby P, Schönbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91:281–291. doi: 10.1161/01.res.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Pober JS. Chronic rejection. Immunity. 2001;14:387–397. doi: 10.1016/s1074-7613(01)00119-4. [DOI] [PubMed] [Google Scholar]
- 3.Rao DA, Pober JS. Endothelial injury, alarmins, and allograft rejection. Crit Rev Immunol. 2008;28:229–248. doi: 10.1615/critrevimmunol.v28.i3.40. [DOI] [PubMed] [Google Scholar]
- 4.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 5.Grattan MT, Moreno-Cabral CE, Starnes VA, Oyer PE, Stinson EB, Shumway NE. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA. 1989;261:3561–3566. [PubMed] [Google Scholar]
- 6.Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–1161. [PubMed] [Google Scholar]
- 7.Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T, Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Karikó K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- 11.Okabe Y, Kawane K, Akira S, Taniguchi T, Nagata S. Toll-like receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. J Exp Med. 2005;202:1333–1339. doi: 10.1084/jem.20051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawane K, Ohtani M, Miwa K, Kizawa T, Kanbara Y, Yoshioka Y, Yoshikawa H, Nagata S. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature. 2006;443:998–1002. doi: 10.1038/nature05245. [DOI] [PubMed] [Google Scholar]
- 13.Cavassani KA, Ishii M, Wen H, Schaller MA, Lincoln PM, Lukacs NW, Hogaboam CM, Kunkel SL. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205:2609–2621. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinet W, Knaapen MW, De Meyer GR, Herman AG, Kockx MM. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation. 2002;106:927–932. doi: 10.1161/01.cir.0000026393.47805.21. [DOI] [PubMed] [Google Scholar]
- 15.Martinet W, de Meyer GR, Herman AG, Kockx MM. Reactive oxygen species induce RNA damage in human atherosclerosis. Eur J Clin Invest. 2004;34:323–327. doi: 10.1111/j.1365-2362.2004.01343.x. [DOI] [PubMed] [Google Scholar]
- 16.Arkonac B, Mauck KA, Chou S, Hosenpud JD. Low multiplicity cytomegalovirus infection of human aortic smooth muscle cells increases levels of major histocompatibility complex class I antigens and induces a proinflammatory cytokine milieu in the absence of cytopathology. J Heart Lung Transplant. 1997;16:1035–1045. [PubMed] [Google Scholar]
- 17.Yang X, Murthy V, Schultz K, Tatro JB, Fitzgerald KA, Beasley D. Toll-like receptor 3 signaling evokes a proinflammatory and proliferative phenotype in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;291:H2334–H2343. doi: 10.1152/ajpheart.00252.2006. [DOI] [PubMed] [Google Scholar]
- 18.Tellides G, Pober JS. Interferon-gamma axis in graft arteriosclerosis. Circ Res. 2007;100:622–632. doi: 10.1161/01.RES.0000258861.72279.29. [DOI] [PubMed] [Google Scholar]
- 19.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 20.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 21.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 22.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 23.Ranjbaran H, Sokol SI, Gallo A, Eid RE, Iakimov AO, D’Alessio A, Kapoor JR, Akhtar S, Howes CJ, Aslan M, Pfau S, Pober JS, Tellides G. An inflammatory pathway of IFN-gamma production in coronary atherosclerosis. J Immunol. 2007;178:592–604. doi: 10.4049/jimmunol.178.1.592. [DOI] [PubMed] [Google Scholar]
- 24.Tellides G, Tereb DA, Kirkiles-Smith NC, Kim RW, Wilson JH, Schechner JS, Lorber MI, Pober JS. Interferon-gamma elicits arteriosclerosis in the absence of leukocytes. Nature. 2000;403:207–211. doi: 10.1038/35003221. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Bai Y, Qin L, Zhang P, Yi T, Teesdale SA, Zhao L, Pober JS, Tellides G. Interferon-gamma induces human vascular smooth muscle cell proliferation and intimal expansion by phosphatidylinositol 3-kinase dependent mammalian target of rapamycin raptor complex 1 activation. Circ Res. 2007;101:560–569. doi: 10.1161/CIRCRESAHA.107.151068. [DOI] [PubMed] [Google Scholar]
- 26.Niimi K, Asano K, Shiraishi Y, Nakajima T, Wakaki M, Kagyo J, Takihara T, Suzuki Y, Fukunaga K, Shiomi T, Oguma T, Sayama K, Yamaguchi K, Natori Y, Matsumoto M, Seya T, Yamaya M, Ishizaka A. TLR3-mediated synthesis and release of eotaxin-1/CCL11 from human bronchial smooth muscle cells stimulated with double-stranded RNA. J Immunol. 2007;178:489–495. doi: 10.4049/jimmunol.178.1.489. [DOI] [PubMed] [Google Scholar]
- 27.Livengood AJ, Wu CC, Carson DA. Opposing roles of RNA receptors TLR3 and RIG-I in the inflammatory response to double-stranded RNA in a Kaposi’s sarcoma cell line. Cell Immunol. 2007;249:55–62. doi: 10.1016/j.cellimm.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalali BN, Köllisch G, Mages J, Müller T, Bauer S, Wagner H, Ring J, Lang R, Mempel M, Ollert M. Double-stranded RNA induces an antiviral defense status in epidermal keratinocytes through TLR3-, PKR-, and MDA5/RIG-I-mediated differential signaling. J Immunol. 2008;181:2694–2704. doi: 10.4049/jimmunol.181.4.2694. [DOI] [PubMed] [Google Scholar]
- 29.Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, Núñez G. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 30.Malathi K, Dong B, Gale M, Jr, Silverman RH. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature. 2007;448:816–819. doi: 10.1038/nature06042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai Y, Ahmad U, Wang Y, Li JH, Choy JC, Kim RW, Kirkiles-Smith N, Maher SE, Karras JG, Bennett CF, Bothwell AL, Pober JS, Tellides G. Interferon-gamma induces X-linked inhibitor of apoptosis-associated factor-1 and Noxa expression and potentiates human vascular smooth muscle cell apoptosis by STAT3 activation. J Biol Chem. 2008;283:6832–6842. doi: 10.1074/jbc.M706021200. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki K, Mori A, Ishii KJ, Saito J, Singer DS, Klinman DM, Krause PR, Kohn LD. Activation of target-tissue immune-recognition molecules by double-stranded polynucleotides. Proc Natl Acad Sci U S A. 1999;96:2285–2290. doi: 10.1073/pnas.96.5.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, Ohba Y, Taniguchi T. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 34.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caron G, Duluc D, Frémaux I, Jeannin P, David C, Gascan H, Delneste Y. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J Immunol. 2005;175:1551–1557. doi: 10.4049/jimmunol.175.3.1551. [DOI] [PubMed] [Google Scholar]
- 36.Lundberg AM, Drexler SK, Monaco C, Williams LM, Sacre SM, Feldmann M, Foxwell BM. Key differences in TLR3/poly I:C signaling and cytokine induction by human primary cells: a phenomenon absent from murine cell systems. Blood. 2007;110:3245–3252. doi: 10.1182/blood-2007-02-072934. [DOI] [PubMed] [Google Scholar]
- 37.Weber F, Wagner V, Rasmussen SB, Hartmann R, Paludan SR. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J Virol. 2006;80:5059–5064. doi: 10.1128/JVI.80.10.5059-5064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gantier MP, Tong S, Behlke MA, Xu D, Phipps S, Foster PS, Williams BR. TLR7 is involved in sequence-specific sensing of single-stranded RNAs in human macrophages. J Immunol. 2008;180:2117–2124. doi: 10.4049/jimmunol.180.4.2117. [DOI] [PubMed] [Google Scholar]
- 39.Prens EP, Kant M, van Dijk G, van der Wel LI, Mourits S, van der Fits L. IFN-alpha enhances poly-IC responses in human keratinocytes by inducing expression of cytosolic innate RNA receptors: relevance for psoriasis. J Invest Dermatol. 2008;128:932–938. doi: 10.1038/sj.jid.5701087. [DOI] [PubMed] [Google Scholar]
- 40.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 41.Yang D, Chen Q, Rosenberg HF, Rybak SM, Newton DL, Wang ZY, Fu Q, Tchernev VT, Wang M, Schweitzer B, Kingsmore SF, Patel DD, Oppenheim JJ, Howard OM. Human ribonuclease A superfamily members, eosinophil-derived neurotoxin and pancreatic ribonuclease, induce dendritic cell maturation and activation. J Immunol. 2004;173:6134–6142. doi: 10.4049/jimmunol.173.10.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ng EK, Tsui NB, Lam NY, Chiu RW, Yu SC, Wong SC, Lo ES, Rainer TH, Johnson PJ, Lo YM. Presence of filterable and nonfilterable mRNA in the plasma of cancer patients and healthy individuals. Clin Chem. 2002;48:1212–1217. [PubMed] [Google Scholar]
- 43.Schaub FJ, Han DK, Liles WC, Adams LD, Coats SA, Ramachandran RK, Seifert RA, Schwartz SM, Bowen-Pope DF. Fas/FADD-mediated activation of a specific program of inflammatory gene expression in vascular smooth muscle cells. Nat Med. 2000;6:790–796. doi: 10.1038/77521. [DOI] [PubMed] [Google Scholar]
- 44.Fries DM, Lightfoot R, Koval M, Ischiropoulos H. Autologous apoptotic cell engulfment stimulates chemokine secretion by vascular smooth muscle cells. Am J Pathol. 2005;167:345–353. doi: 10.1016/S0002-9440(10)62980-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geng YJ, Libby P. Evidence for apoptosis in advanced human atheroma. Colocalization with interleukin-1 beta-converting enzyme. Am J Pathol. 1995;147:251–266. [PMC free article] [PubMed] [Google Scholar]
- 46.Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, Bennett MR. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 47.Schultz K, Murthy V, Tatro JB, Beasley D. Endogenous interleukin-1 alpha promotes a proliferative and proinflammatory phenotype in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2007;292:H2927–H2934. doi: 10.1152/ajpheart.00700.2006. [DOI] [PubMed] [Google Scholar]
- 48.Rao DA, Eid RE, Qin L, Yi T, Kirkiles-Smith NC, Tellides G, Pober JS. Interleukin (IL)-1 promotes allogeneic T cell intimal infiltration and IL-17 production in a model of human artery rejection. J Exp Med. 2008;205:3145–3158. doi: 10.1084/jem.20081661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clarke MC, Talib S, Figg NL, Bennett MR. Vascular smooth muscle cell apoptosis induces interleukin-1-directed inflammation: effects of hyperlipidemia-mediated inhibition of phagocytosis. Circ Res. 2010;106:363–372. doi: 10.1161/CIRCRESAHA.109.208389. [DOI] [PubMed] [Google Scholar]