Figure 5:
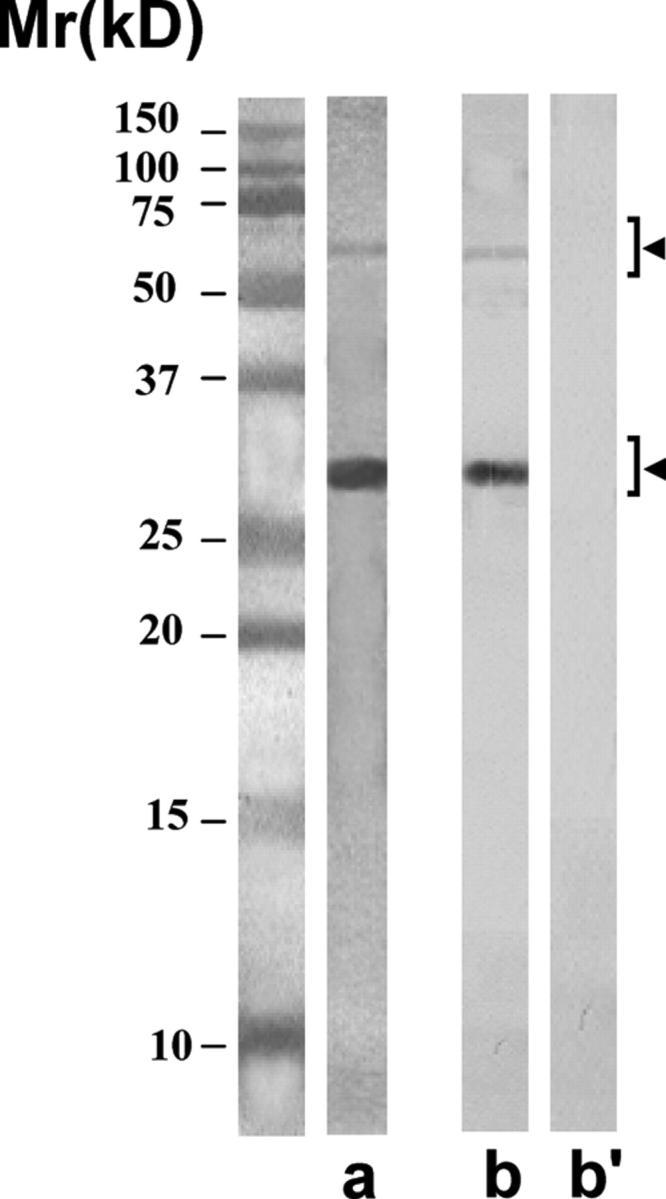
Purity of the scFv antibodies isolated by using anti-E-Tag antibody column
The purified scFv antibodies showed predominantly the expected single protein band of ∼28 kDa in SDS–PAGE after staining with silver nitrate (lane a). On freezing, the antibodies had a tendency to polymerize into dimeric form of ∼56 kDa, and sometimes into trimeric form of ∼84 kDa (not shown). All these forms were specifically recognized by the anti-E-Tag monoclonal antibody (lane b) and not by the myeloma control monoclonal antibody (lane b’) in the western blot procedure.
