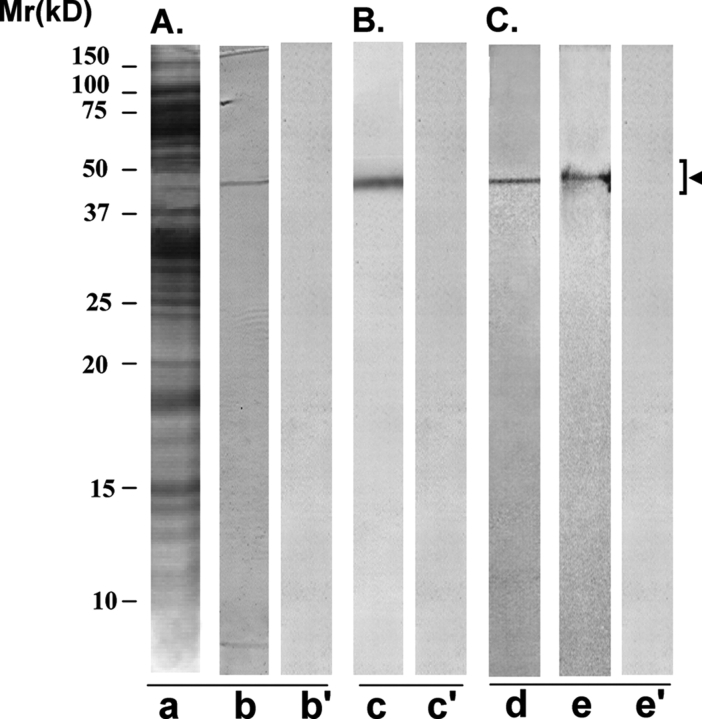
Figure 6:
Immunoreactivity pattern of AFA-1 scFv antibody with HSE and FA-1 antigen
The purified scFv antibody was examined for its immunoreactivity with LIS-solubilized HSE (A and B) and purified cognate human sperm FA-1 antigen (C), using western blot (A and C) and immunoprecipitation (B) procedures. (A) SDS–PAGE of HSE revealed several protein bands of various molecular identities after silver staining (lane a). AFA-1 scFv antibody specifically recognized a protein band of 50 ± 4 kDa, corresponding to FA-1 antigen on western blot of HSE (lane b). Control scFv antibody did not react with any specific band on the western blot (lane b’). (B) AFA-1 scFv antibody Sepharose 4B immunobeads reacted with a specific protein in HSE that on elution with glycine–HCl (0.1 M, pH 2.8) showed a single band of 50 ± 4 kDa, corresponding to FA-1 antigen, in SDS–PAGE (lane c). Control scFv antibody Sepharose 4B immunobeads did not react with any protein in HSE (lane c’). (C) The cognate FA-1 antigen purified from HSE using immunoaffinity column involving mouse monoclonal antibody MA-24 showed a single band of 50 ± 4 kDa in SDS–PAGE (lane d) that was specifically recognized by AFA-1 scFv antibody (lane e) and not by the control scFv antibody (lane e’) in the western blot procedure.