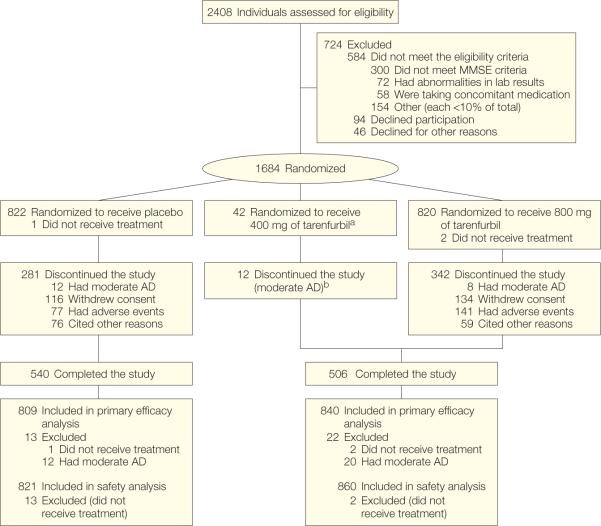
Figure 1.
Participant Flow Chart
aPatients originally assigned to receive twice daily 400 mg of tarenflurbil were incorporated into the twice daily 800-mg group.
bExcluded from the main efficacy analyses using the intent-to-treat population but were included in the safety analyses. Rates of adverse events leading to study discontinuation are based on the intent-to-treat population. The numbers differ from those in the text, which are based on the safety population.