FIGURE 8.
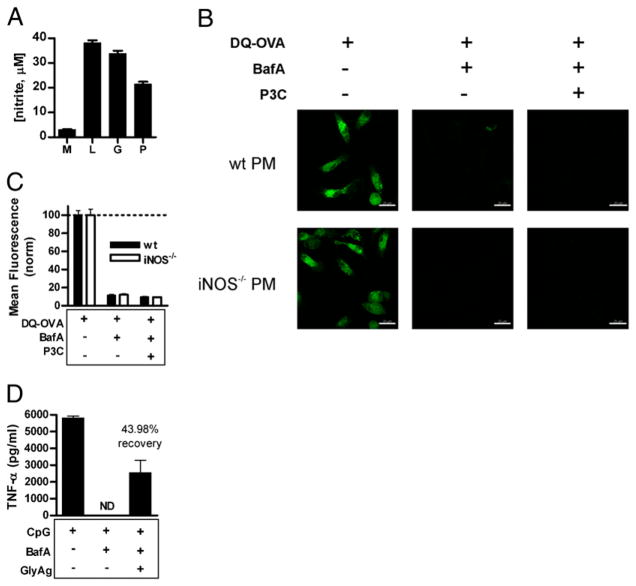
P3C-mediated TLR2 signaling and NO production is inadequate to induce acidification, yet GlyAg oxidation promotes CpG-mediated TLR9 signaling (A). To verify P3C-induced NO production, RAW macrophages were stimulated with 15 nM P3C (P), 100 ng/ml LPS (L), 100 μg/ml GlyAg (G), or left untreated (M); spent media were analyzed for nitrite concentration at 48 h as determined by the Griess reagent (n = 3). All Ags stimulated significant NO production. B, PMs were treated with 40 nM BafA then 15 nM P3C overnight, washed, and incubated with 50 μg/ml DQ-OVA for 25 min. Multiple confocal images (63×) for both WT and iNOS−/− PMs and each treatment condition were taken, which showed little to no green signal and therefore no OVA processing in BafA-treated cells given P3C. Scale bar, 20 μm. C, Images were analyzed using the Leica Application Suite for ROI mean intensity values (n ≥ 40 ROIs per sample; error bars are SEM) and normalized to the respective DQ-OVA–only positive control samples. Despite P3C-mediated, TLR2-dependent synthesis of NO (not shown), P3C failed to induce acidification in PMs over the BafA-treatment, suggesting that both NO and NO oxidation of GlyAg were collectively necessary to achieve acidification. D, RAW macrophages were treated with 40 nM BafA overnight to inhibit TLR9 signaling and then stimulated with 1 μg/ml CpG with and without 50 μg/ml GlyAg. Supernatants were removed 24 h later, and the amount of TNFα produced was determined by ELISA. TNFα production was normalized to the control (CpG without BafA) and compared with the cells incubated with and without GlyAg and BafA. As expected, BafA eliminated TLR9 signaling, but GlyAg oxidation was able to restore 43.98% of the TNFα production induced by CpG and TLR9. Error bars represent SEM. ND, not detected.