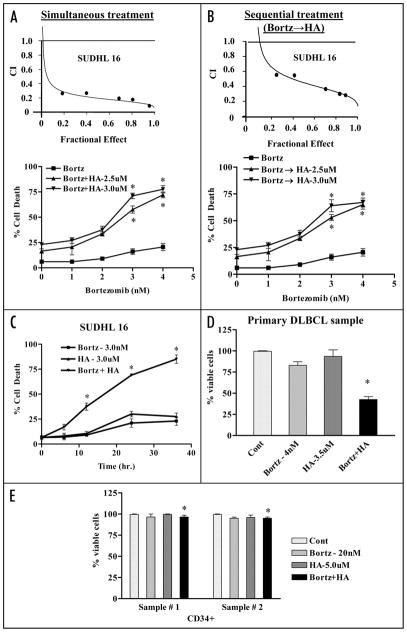
Figure 1.
Co-treatment of bortezomib and Bcl-2 antagonist leads to synergistic induction of cell death in DLBCL cells in a time dependent manner but not in normal hematopoietic cells. SUDHL16 cells were treated with indicated concentration of bortezomib ± HA14-1. (A) simultaneously or (B) sequentially (8 h bortezomib pretreatment followed by HA14-1) for a total of 36 h, after which cell death was monitored by flow cytometry with 7AAD staining. Insets (A and B): Fractional Effect (FA) values were determined by comparing results to those of untreated controls, and Median Dose Effect analysis was employed to characterize the nature of the interaction. Combination Index (C.I.) values less than 1.0 denote a synergistic interaction. Two additional studies yielded equivalent results. (C) SUDHL6 cells were treated with bortezomib ± HA14-1 at the indicated concentrations for various intervals, and cell death was determined by flow-cytometry with 7AAD staining (D) Primary human bone marrow DLBCL cells were isolated as described in Methods and suspended in medium containing 10% FCS at a cell density of 0.75 × 106/ml cells in the presence of 4 nM bortezomib ± 3.5 uM of HA14-1 for 14 h. At the end of drug exposure, apoptotic cells were monitored by Annexin/PI staining. Apoptotic cell death for controls was <25–20%. The percentage of non apoptotic cells were considered to represent the viable fraction, and values were normalized to controls. (E) CD34+ cells obtained from the bone marrow of two patients undergoing routine diagnostic procedures for non-myeloid hematologic disorders were isolated by an immunomagnetic bead separation technique as described in Methods and exposed to bortezomib (20 nM) ± HA14-1 (5.0 μM) for 48 h. At the end of this period, the percentage of apoptotic cells was determined by Annexin V/PI staining and flow cytometry. The percentage of viable cells in each sample was normalized to controls. Values represent the means ± S.D. for triplicate determination. For (A–C), * = significantly greater than bortezomib alone; p < 0.01. For (D), * = not significantly different from values for untreated controls; p > 0.05.