Abstract
BACKGROUND:
Interaction of the receptors for advanced glycation end products (RAGEs) with advanced glycation end products (AGEs) results in expression of inflammatory mediators (tumor necrosis factor-alpha [TNF-α] and soluble vascular cell adhesion molecule-1 [sVCAM-1]), activation of nuclear factor-kappa B and induction of oxidative stress – all of which have been implicated in atherosclerosis. Soluble RAGE (sRAGE) acts as a decoy for the RAGE ligand and is protective against atherosclerosis.
OBJECTIVES:
To determine whether levels of serum sRAGE are lower, and whether levels of serum AGEs, TNF-α and sVCAM-1 are higher in non-ST elevation myocardial infarction (NSTEMI) patients than in healthy control subjects; and whether sRAGE or the ratio of AGEs to sRAGE (AGEs/sRAGE) is a predictor/biomarker of NSTEMI.
METHODS:
Serum levels of sRAGE, AGEs, TNF-α and sVCAM-1 were measured in 46 men with NSTEMI and 28 age- and sex-matched control subjects. Angiography was performed in the NSTEMI patients.
RESULTS:
sRAGE levels were lower, and levels of AGEs, TNF-α, sVCAM-1 and AGEs/sRAGE were higher in NSTEMI patients than in control subjects. sRAGE levels were negatively correlated with the number of diseased coronary vessels, serum AGEs, AGEs/sRAGE, TNF-α and sVCAM-1. The sensitivity of the AGEs/sRAGE test is greater than that of the sRAGE test, while the specificity and predictive values of the sRAGE test are greater than those of the AGEs/sRAGE test for identifying NSTEMI patients.
CONCLUSIONS:
Serum levels of sRAGE were low in NSTEMI patients, and were negatively correlated with extent of lesion, inflammatory mediators, AGEs and AGEs/sRAGE. Both sRAGE and AGEs/sRAGE may serve as biomarkers/predictors for identifying NSTEMI patients.
Keywords: Advanced glycation end product, Non-ST elevation myocardial infarction, Soluble receptor for advanced glycation end products, Soluble vascular cell adhesion molecule-1, Tumor necrosis factor-alpha
Advanced glycation end products (AGEs) are a heterogeneous group of irreversible adducts resulting from non-enzymatic glycation and oxidation of proteins, lipids and nucleic acids (1,2). AGEs act on cell receptors for AGEs (RAGEs). There are three forms of RAGEs (2–5) – full-length, N-truncated and C-truncated soluble RAGEs (sRAGE). The interaction of full-length RAGE with AGEs leads to increased expression of adhesion molecules, including soluble vascular cell adhesion molecule-1 (sVCAM-1) and the cytokine tumor necrosis factor-alpha (TNF-α) (2,6,7); activation of nuclear factor-kappa B (6), which in turn leads to increased expression of proinflammatory genes for adhesion molecules and cytokines (2); and generation of oxygen radicals (8,9). sRAGE circulates in the plasma (4) and acts as a decoy for RAGE ligands, competing with full-length RAGE for ligand binding (10). It has a protective role by preventing the activation of full-length RAGE.
Adhesion molecules, cytokines and oxygen radicals are involved in atherosclerosis, progression of lesions and lesion instability (11–14). The AGEs and RAGE axis has been implicated in the pathogenesis of atherosclerosis in diabetes (15–17). sRAGE in animal models reduces atherosclerotic lesions, aortic vascular cell adhesion molecule-1 and tissue factor (18–20). The proximate cause of acute coronary syndrome (ACS) is thrombosis and the principal underlying cause is atherosclerosis. Because the combination of AGEs, RAGEs and sRAGE determines the extent of vascular injury, the measurement of these factors is appropriate for determining vascular complications. However, it is not possible to measure RAGE in humans, which is on the cell surface of the artery.
It is hypothesized that non-ST elevation myocardial infarction (NSTEMI) patients have lower levels of serum sRAGE and/or higher levels of AGEs, and a higher ratio of AGEs to sRAGE (AGEs/sRAGE) than healthy subjects. Because the interaction of RAGE with AGEs results in increased expression of cytokines and adhesion molecules, and because sRAGE neutralizes AGEs, low sRAGE levels would be associated with high levels of TNF-α and sVCAM-1. Therefore, the main objectives of the present study are to determine whether levels of serum sRAGE are lower, and whether levels of serum AGEs and AGEs/sRAGE are higher in NSTEMI patients than in healthy subjects; whether low levels of serum sRAGE, and high levels of serum AGEs and AGEs/sRAGE are associated with high levels of serum TNF-α and sVCAM-1; whether the number of diseased coronary arteries is negatively correlated with serum sRAGE and positively correlated with serum TNF-α, sVCAM-1, AGEs and AGEs/sRAGE; and whether the level of sRAGE or AGEs/sRAGE is a better biomarker/predictor of NSTEMI.
METHODS
Study population
The present study comprised 46 consecutive male NSTEMI patients admitted to the Royal University Hospital, Saskatoon, Saskatchewan, and 28 male age-matched control subjects. Patients with NSTEMI and discrete de novo localized lesions in single or multiple vessels, and who were between 50 and 70 years of age were included in the study. Patients with acute myocardial infarction within the previous five days, bypass surgery, inflammatory disease, valvular heart disease, Alzheimer’s disease, diabetes and any current smoking habits were excluded from the study.
The selected control subjects had no history of angina or other heart disease, a normal resting electrocardiogram, were normotensive, and had no current smoking habits, Alzheimer’s disease, diabetes or inflammatory disease. Cigarette smokers were defined as having smoked ‘ever’, rather than being current or former smokers. Hypertension was defined as blood pressure of greater than 140/90 mmHg, or the use of antihypertensive medications. The study protocol was approved by the Ethics Committee for Human Studies at the University of Saskatchewan (Saskatoon) and Royal University Hospital. Written informed consent was obtained from each participant.
Angiographic analysis
Angiography was performed and the angiograms were analyzed by two observers blinded to the clinical characteristics of the patients. Reference diameter, minimal lumen diameter, percentage of stenosis and lesion length were measured using a semiautomated edge counter detection computer analysis system (QCACMS Version 4, Medis Medical Imaging Systems Inc, The Netherlands). Coronary angiography showed that 16 patients had one-vessel disease (1VD), 15 patients had two-vessel disease (2VD) and 15 patients had three-vessel disease or more (3VD). The number of diseased vessels was used for correlation with biochemical parameters (sRAGE, AGEs, AGEs/sRAGE, TNF-α and sVCAM-1).
Biochemical measurements
Blood samples were collected for measurement of serum AGEs, sRAGE, TNF-α, sVCAM-1, lipids, creatine kinase, cardiac troponin I, glucose and glycated hemoglobin. Serum total sRAGE levels were measured using a commercially available ELISA kit (R&D Systems, USA). TNF-α was measured using an ELISA kit (R&D Systems). sVCAM-1 was measured using an ELISA kit (Abcam Inc, USA). AGE levels were measured using a human AGE ELISA kit (BIOPCR, Beijing Zhonghao Shidai Co Ltd, China). All measurements were made according to the manufacturers’ protocols. Serum total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol, triglycerides, glucose and creatine kinase were measured on a Beckman DxC-800 (Beckman Coulter Inc, USA) using standard laboratory protocol. Troponin I was measured using an Abbott Architect (i2000-SR, Abbott Laboratories, USA)
Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy of the sRAGE and AGEs/sRAGE tests in identifying NSTEMI patients were calculated as described by Glas et al (21).
Statistical analysis
The sample size was calculated, using a two-sided Satterthwaite t test, to have 95% power and a 5% alpha level. Data are reported as mean ± SE. The data were compared between the two groups using the two-tailed unpaired Student t test. Single linear univariate correlations (Pearson’s correlation coefficients) were calculated to evaluate the relationships between circulating sRAGE levels and the following variables: serum TNF-α, sVCAM-1, AGEs and AGEs/sRAGE. The serum levels of sRAGE, AGEs, AGEs/sRAGE, TNF-α and sVCAM-1 in 1VD, 2VD and 3VD were compared with control values. P<0.05 was considered significant.
RESULTS
The baseline characteristics of the NSTEMI and control subjects are shown in Table 1. There were no significant differences in age, body mass index, diastolic arterial blood pressures and serum glucose between the two groups. However, the levels of TC, low-density lipoprotein cholesterol, triglycerides, risk ratio (TC/HDL-C) and systolic arterial blood pressure were higher, and levels of HDL-C were lower in NSTEMI patients than in control subjects. All NSTEMI patients were smokers. None of the control subjects were hypertensive; however, 76% of the NSTEMI patients were hypertensive.
TABLE 1.
Baseline characteristics of the study subjects
| Control subjects | NSTEMI subjects | |
|---|---|---|
| Subjects, n | 28 | 46 |
| Age, years | 60±2.0 | 63.8±1.5 |
| Smoking | No | Yes |
| Body mass index, kg/m2 | 25±1.5 | 29±0.7 |
| Systolic BP, mmHg | 125±4.3 | 150±15.8* |
| Diastolic BP, mmHg | 78±2.7 | 68±10.4 |
| Hypertension, n | 0 | 35 |
| TC, mmol/L | 4.40±0.25 | 6.04±0.25* |
| HDL-C, mmol/L | 1.35±0.14 | 0.92±0.30* |
| LDL-C, mmol/L | 2.78±0.10 | 4.33±0.33* |
| Triglycerides, mmol/L | 2.19±0.30 | 2.60±0.32 |
| Risk ratio (TC/HDL-C) | 3.89±0.33 | 7.62±0.55* |
| Glucose, mmol/L | 5.78±0.32 | 5.55±0.21 |
| Cardiac troponin I, μg/L | 2.70±0.48 | |
| Creatine kinase, U/L | 383±55.8 |
Results are expressed as mean ± SE unless otherwise indicated.
P<0.05, control versus non-ST elevation myocardial infarction (NSTEMI). BP Blood pressure; HDL-C High-density lipoprotein cholesterol; LDL-C Low-density lipoprotein cholesterol; TC Total cholesterol
Serum sRAGE, AGEs, AGEs/sRAGE, TNF-α and sVCAM-1
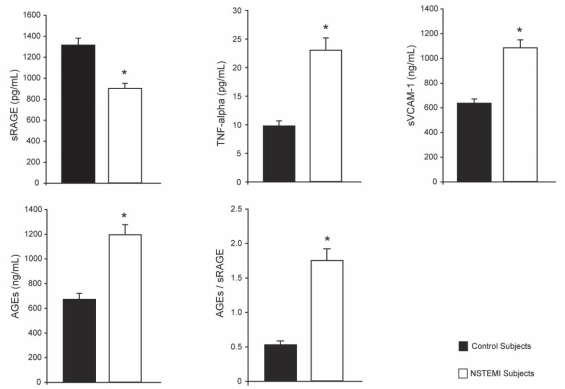
Serum levels of sRAGE, AGEs, AGEs/sRAGE, TNF-α and sVCAM-1 are summarized in Figure 1. The level of sRAGE in the control subjects was 1287.0±41.5 pg/mL. The levels were lower in NSTEMI patients than in control subjects. The levels of AGEs in NSTEMI subjects were higher than in control subjects (1192.5±82.6 ng/mL versus 669.4±47.9 ng/mL). The ratio of AGEs/sRAGE was higher in NSTEMI patients than in control subjects (1.72±0.14 versus 0.54±0.06). The levels of TNF-α in control and NSTEMI subjects were 10.3±0.8 pg/mL and 23.1±2.3 pg/mL, respectively. The levels of sVCAM-1 in control subjects were lower than in NSTEMI patients (651.0±35.5 ng/mL versus 1059.6±70.8 ng/mL).
Figure 1).
Serum levels of soluble receptors for advanced glycation end products (sRAGE), advanced glycation end products (AGEs), AGEs/sRAGE, tumor necrosis factor-alpha (TNF-alpha) and soluble vascular cell adhesion molecule-1 (sVCAM-1) in control subjects and non-ST elevation myocardial infarction (NSTEMI) patients. Results are expressed as mean ± SE. *P<0.05, control subjects versus NSTEMI patients
Relation of sRAGE, AGEs, TNF-α and sVCAM-1 with number of diseased vessels
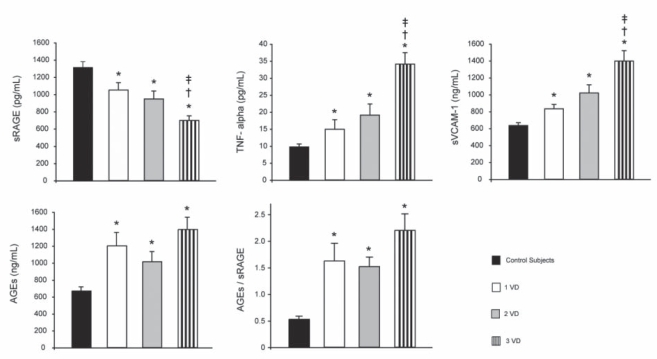
The levels of serum sRAGE, AGEs, AGEs/sRAGE, TNF-α and sVCAM-1 in relation to the number of diseased vessels are summarized in Figure 2. The levels of sRAGE in patients with 1VD, 2VD and 3VD were 21%, 26% and 47% lower, respectively, than in the control subjects. The values were not different between patients with 1VD and 2VD; however, they were lower in patients with 3VD than in patients with 1VD and 2VD. The levels of AGEs were higher to a similar extent in patients with diseased vessels than in the control subjects. The AGEs/sRAGE values in patients with 1VD, 2VD and 3VD were similar but higher than in the controls. The levels of sVCAM-1 in patients with 1VD, 2VD and 3VD were 30%, 57% and 114% higher, respectively, than in the control subjects. The sVCAM-1 values were not different between 1VD and 2VD patients; however, they were higher in 3VD patients than in both 1VD and 2VD patients. The levels of TNF-α in patients with 1VD, 2VD and 3VD were 49%, 85% and 140% higher, respectively, than in the control subjects. The TNF-α levels were not significantly different in patients with 1VD and 2VD, but the levels were higher in patients with 3VD than in patients with 1VD and 2VD.
Figure 2).
Serum levels of soluble receptors for advanced glycation end products (sRAGE), advanced glycation end products (AGEs), AGEs/sRAGE, soluble vascular cell adhesion molecule-1 (sVCAM-1) and tumor necrosis factor-alpha (TNF-alpha) in control subjects and non-ST elevation myocardial infarction (NSTEMI) patients with one-vessel (1VD), two-vessel (2VD), and three-vessel disease or more (3VD). The results are expressed as mean ± SE. *P<0.05, control subjects versus NSTEMI patients with 1VD, 2VD or 3VD; †P<0.05, 1VD versus 2VD or 3VD; ‡P<0.05, 2VD versus 3VD
Correlation of sRAGE to TNF-α, sVCAM-1, AGEs and AGEs/sRAGE
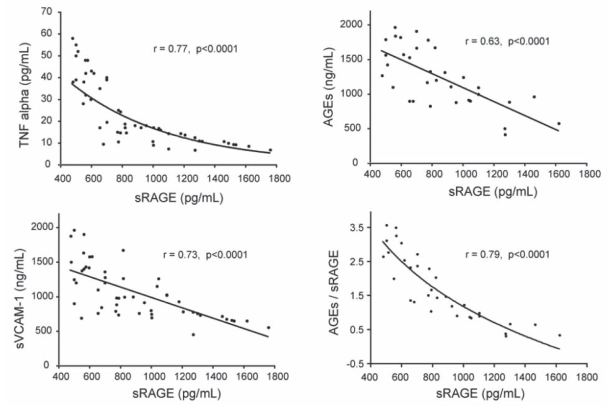
The levels of serum sRAGE negatively correlated with the levels of serum AGEs, AGEs/sRAGE, TNF-α and sVCAM-1 (Figure 3).
Figure 3).
Correlation of soluble receptors for advanced glycation end products (sRAGE) with advanced glycation end products (AGEs), tumor necrosis factor-alpha (TNF-alpha) and soluble vascular cell adhesion molecule-1 (sVCAM-1) in non-ST elevation myocardial infarction patients
Sensitivity, specificity, predictive value and accuracy of the sRAGE test
The mean – 2SD (848.7 pg/mL) for sRAGE and mean + 2SD (0.92) for AGEs/sRAGE, which cover 95% of the control subjects, were used as the cut-off points. Any values of sRAGE above 848.7 pg/mL and values of AGEs/sRAGE below 0.92 were considered normal. The sensitivity, specificity, PPV, NPV and accuracy of the sRAGE biomarker test were 59%, 100%, 100%, 100% and 74%, respectively. The sensitivity, specificity, PPV, NPV and accuracy of the AGEs/sRAGE test were 85%, 91%, 97%, 67% and 86%, respectively. The sensitivity of the AGEs/sRAGE test appears to be greater than that of sRAGE, while the specificity and predictive values of sRAGE are greater than those of AGEs/sRAGE.
DISCUSSION
sRAGE
The present study is the first to show that serum sRAGE levels were lower in NSTEMI patients than in control subjects, and that the levels were inversely related to the number of diseased vessels. Falcone et al (22) showed that the concentration of sRAGE in the plasma of patients with coronary artery disease (CAD) was lower (median [interquartile range]: 966 pg/mL [658 pg/mL to 1372 pg/mL]) than in control subjects (1335 pg/mL [936 pg/mL to 1954 pg/mL]), which was comparable with our results. However, patients with acute coronary syndrome (ACS) were excluded from their study. ACS is divided into ST elevation myocardial infarction (STEMI), NSTEMI and unstable angina. These three types of ACS have few differences in clinical characteristics, treatment options, prognosis and outcomes (23,24).
AGEs
We demonstrated that serum AGEs levels were elevated in NSTEMI patients compared with control subjects, and that the levels were positively correlated with the number of diseased coronary arteries, and negatively correlated with serum sRAGE. Basta et al (25) showed that plasma Nɛ-(carboxymethyl) lysine levels were elevated on day 1 after coronary stenting in patients with CAD and were significantly higher in multivessel stent implants than in single-vessel stents compared with pre-stent levels. However, they did not compare the pre-stent levels of Nɛ-(carboxymethyl) lysine with those of healthy control subjects. Plasma levels of pentosidine markers of AGEs were associated with coronary artery calcification in hemodialysis patients (26). Nakamura et al (27) reported a positive correlation between sRAGE and AGEs, which is contrary to the present findings. This difference could be due to the difference in the study subjects. The Nakamura study subjects were diabetic patients with CAD, while our study involved nondiabetic NSTEMI patients. They reported higher levels of sRAGE in their CAD patients than in the healthy control subjects. Yamagishi et al (28) have shown that serum sRAGE levels were positively associated with AGEs levels in the nondiabetic general population. These differences were not likely due to the method of biochemical measurement because the same kits for sRAGE measurement were used in the present study.
AGEs/sRAGE
The present study is the first to show that the AGEs/sRAGE ratio was higher in NSTEMI patients than in control subjects, and was positively correlated with the number of diseased vessels and negatively correlated with serum sRAGE levels.
TNF-α
In the present study, serum levels of TNF-α and sVCAM-1 were elevated in NSTEMI patients compared with control subjects. Also, there were inverse correlations between sRAGE, and TNF-α and sVCAM-1. There have been no similar studies performed previously in NSTEMI patients. However, there are reports of elevated levels of TNF-α in STEMI patients (29–31). Individuals carrying the TNF-α −308 AG + AA genotypes are significantly more represented among acute myocardial infarction patients affected by STEMI than among NSTEMI patients and healthy controls (31). Correlations of sRAGE with TNF-α in NSTEMI patients have not been reported previously. However, Nakamura et al (27) reported a positive correlation between sRAGE and TNF-α in type 2 diabetic patients. This discrepancy may be due to the different patient population.
sVCAM-1
In the present study, serum levels of sVCAM-1 were elevated in NSTEMI patients compared with control subjects, and the levels were inversely related to the serum sRAGE levels. The present study is the first of its kind in NSTEMI patients. In another study (32), the serum concentration of sVCAM-1 in STEMI patients varied from 1391±38 ng/mL (nighttime) to 1200±43 ng/mL (daytime). Type 2 diabetic patients with STEMI, compared with control subjects, had sVCAM-1 levels of 1393.4±865.4 ng/mL versus 573.3±226.1 ng/mL, respectively (33). These values are similar to the values in the present study with NSTEMI patients and control subjects (1059.62±71 ng/mL versus 651±35.5 ng/mL, respectively). There is one recent study in which the sVCAM-1 level in STEMI patients was 496±34 ng/mL, which is lower than the other studies (34).
sRAGE and atherosclerosis
The results demonstrated that low serum levels of sRAGE were associated with high levels of serum TNF-α and sVCAM-1 in NSTEMI patients. The possibility exists that CAD in these patients may be related to an increase in the levels of serum TNF-α and sVCAM-1. TNF-α may induce atherosclerosis in many ways. TNF-α stimulates superoxide (O2–) production in neutrophils and endothelial cells via NADPH oxidase (35), xanthine oxidase (36) and nitric oxide (NO) synthase (37). TNF-α activates nuclear factor-kappa B, which regulates the expression of genes involved in inflammation and oxidative stress (38). TNF-α decreases the bioavailability of NO by diminishing its production (39) and enhancing its removal (40). Oxidative stress (14,41,42) and deficiency of NO (43) have been implicated in the pathophysiology of atherosclerosis.
Adhesion molecules are involved in the development of atherosclerosis (11,14). In patients with established disease, sVCAM-1 is a better marker of the extent and severity of atherosclerosis (44). The increased levels of sVCAM-1 in NSTEMI patients in the present study suggest the involvement of sVCAM-1 in CAD.
Low levels of serum sRAGE in NSTEMI patients may be associated with CAD. In support of this finding, it has been reported that balloon injury in the carotid artery was associated with neointimal hyperplasia and increased levels of AGEs and RAGE in the lesion (17). Administration of sRAGE before balloon injury reduced neointimal growth in this study. Sakaguchi et al (18) also reported that endothelial injury in mice was associated with neointimal expansion and deposition of AGEs in the expanding neointima. sRAGE administration decreased neointimal expansion, and smooth muscle cell proliferation and migration in this study. sRAGE treatment significantly reduced atherosclerotic lesions and VCAM-1 in this study. Treatment of diabetic apolipoprotein E-deficient mice with sRAGE completely suppressed atherosclerosis (19,20).
Acknowledgments
This study was supported by grants from the Heart and Stroke Foundation of Saskatchewan and the Pollack Research Foundation, Saskatoon, Saskatchewan. This manuscript forms a part of the PhD thesis of Dr Erick McNair. The authors acknowledge the assistance of Ms Heather Neufeld in biochemical measurements, Dr Hyun J Lim in data analysis and Ms Barbara Raney in the preparation of the manuscript.
REFERENCES
- 1.Thorpe SR, Baynes JW. Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids. 2003;25:275–81. doi: 10.1007/s00726-003-0017-9. [DOI] [PubMed] [Google Scholar]
- 2.Prasad K. Soluble receptor for advanced glycation end products (sRAGE) and cardiovascular disease. Int J Angiol. 2006;15:57–68. [Google Scholar]
- 3.Huttunen HJ, Kuja-Panula J, Sorci G, Agneletti AL, Donato R, Rauvala H. Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J Biol Chem. 2000;275:40096–105. doi: 10.1074/jbc.M006993200. [DOI] [PubMed] [Google Scholar]
- 4.Yonekura H, Yamamoto Y, Sakurai S, et al. Novel splice variants of the receptor for advanced glycation end products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. 2003;370:1097–109. doi: 10.1042/BJ20021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudson BI, Harja E, Moser B, Schmidt AM. Soluble levels of receptor for advanced glycation end products (sRAGE) and coronary artery disease: The next C-reactive protein? Arterioscler Thromb Vasc Biol. 2005;25:879–82. doi: 10.1161/01.ATV.0000164804.05324.8b. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann MA, Drury S, Fu C, et al. RAGE mediates a novel proinflammatory axis: A central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 7.Reznikov LL, Waksman J, Azam T, et al. Effect of advanced glycation end products on endotoxin-induced TNF-alpha, IL-1beta and IL-8 in human peripheral blood mononuclear cells. Clin Nephrol. 2004;61:324–36. doi: 10.5414/cnp61324. [DOI] [PubMed] [Google Scholar]
- 8.Yan SD, Schmidt AM, Anderson GM, et al. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269:9889–97. [PubMed] [Google Scholar]
- 9.Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280:E685–94. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- 10.Geroldi D, Falcone C, Emanuele E. Soluble receptor for advanced glycation end products: From disease marker to potential therapeutic target. Curr Med Chem. 2006;13:1971–8. doi: 10.2174/092986706777585013. [DOI] [PubMed] [Google Scholar]
- 11.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 12.Young JL, Libby P, Schönbeck U. Cytokines in the pathogenesis of atherosclerosis. Thromb Haemost. 2002;88:554–67. [PubMed] [Google Scholar]
- 13.Prasad K. Reduction of serum cholesterol and hypercholesterolemic atherosclerosis in rabbits by secoisolariciresinol diglucoside isolated from flaxseed. Circulation. 1999;99:1355–62. doi: 10.1161/01.cir.99.10.1355. [DOI] [PubMed] [Google Scholar]
- 14.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt AM, Stern D. Atherosclerosis and diabetes: The RAGE connection. Curr Atheroscler Rep. 2000;2:430–6. doi: 10.1007/s11883-000-0082-4. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt AM, Yan SD, Wautier JL, Stern D. Activation of receptor for advanced glycation end products: A mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ Res. 1999;84:489–97. doi: 10.1161/01.res.84.5.489. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Z, Wang K, Penn MS, et al. Receptor for AGE (RAGE) mediates neointimal formation in response to arterial injury. Circulation. 2003;107:2238–43. doi: 10.1161/01.CIR.0000063577.32819.23. [DOI] [PubMed] [Google Scholar]
- 18.Sakaguchi T, Yan SF, Yan SD, et al. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest. 2003;111:959–72. doi: 10.1172/JCI17115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park L, Raman KG, Lee KJ, et al. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation end products. Nat Med. 1998;4:1025–31. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- 20.Bucciarelli LG, Wendt T, Qu W, et al. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E null mice. Circulation. 2002;106:2827–35. doi: 10.1161/01.cir.0000039325.03698.36. [DOI] [PubMed] [Google Scholar]
- 21.Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: A single indicator of test performance. J Clin Epidemiol. 2003;56:1129–35. doi: 10.1016/s0895-4356(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 22.Falcone C, Emanuele E, D’Angelo A, et al. Plasma levels of soluble receptor for advanced glycation end products and coronary artery disease in nondiabetic men. Arterioscler Thromb Vasc Biol. 2005;25:1032–7. doi: 10.1161/01.ATV.0000160342.20342.00. [DOI] [PubMed] [Google Scholar]
- 23.Bode C, Zirlik A. STEMI and NSTEMI: The dangerous brothers. Eur Heart J. 2007;28:1403–4. doi: 10.1093/eurheartj/ehm159. [DOI] [PubMed] [Google Scholar]
- 24.Montalescot G, Dallongeville J, Van Belle E, et al. for the Opera Investigators STEMI and NSTEMI: Are they so different? 1 year outcomes in acute myocardial infarction as defined by the ESC/ACC definition (the OPERA registry) Eur Heart J. 2007;28:1409–17. doi: 10.1093/eurheartj/ehm031. [DOI] [PubMed] [Google Scholar]
- 25.Basta G, Berti S, Cocci F, et al. Plasma N-epsilon-(carboxymethyl) lysine levels are associated with the extent of vessel injury after coronary arterial stenting. Coron Artery Dis. 2008;19:299–305. doi: 10.1097/MCA.0b013e3282fec058. [DOI] [PubMed] [Google Scholar]
- 26.Taki K, Takayama F, Tsuruta Y, Niwa T. Oxidative stress, advanced glycation end product, and coronary artery calcification in hemodialysis patients. Kidney Int. 2006;70:218–24. doi: 10.1038/sj.ki.5000330. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura K, Yamagishi S, Adachi H, et al. Elevation of soluble form of receptor for advanced glycation end products (sRAGE) in diabetic subjects with coronary artery disease. Diabetes Metab Res Rev. 2007;23:368–71. doi: 10.1002/dmrr.690. [DOI] [PubMed] [Google Scholar]
- 28.Yamagishi S, Adachi H, Nakamura K, et al. Positive association between serum levels of advanced glycation end products and the soluble form of receptor for advanced glycation end products in nondiabetic subjects. Metabolism. 2006;55:1227–31. doi: 10.1016/j.metabol.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Gonzálvez M, Ruiz-Ros JA, Pérez-Paredes M, et al. Prognostic value of tumor necrosis factor-alpha in patients with ST-segment elevation acute myocardial infarction. Rev Esp Cardiol. 2007;60:1233–41. doi: 10.1157/13113928. [DOI] [PubMed] [Google Scholar]
- 30.Theroux P, Armstrong PW, Mahaffey KW, et al. Prognostic significance of blood markers of inflammation in patients with ST-segment elevation myocardial infarction undergoing primary angioplasty and effects of pexelizumab, a C5 inhibitor: A substudy of the COMMA trial. Eur Heart J. 2005;26:1964–70. doi: 10.1093/eurheartj/ehi292. [DOI] [PubMed] [Google Scholar]
- 31.Antonicelli R, Olivieri F, Cavallone L, et al. Tumor necrosis factor-alpha gene −308G>A polymorphism is associated with ST-elevation myocardial infarction and with high plasma levels of biochemical ischemia markers. Coron Artery Dis. 2005;16:489–93. doi: 10.1097/00019501-200512000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Dominguez-Rodriguez A, Abreu-Gonzalez P, Garcia-Gonzalez MJ, Samimi-Fard S, Kaski JC, Reiter RJ. Light/dark patterns of soluble vascular cell adhesion molecule-1 in relation to melatonin in patients with ST-segment elevation myocardial infarction. J Pineal Res. 2008;44:65–9. doi: 10.1111/j.1600-079X.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- 33.Milosz D, Czupryniak L, Saryusz-Wolska M, et al. Adiponectinemia, inflammatory process activity, and endothelial dysfunction in patients with type 2 diabetes and acute coronary syndrome with ST elevation in relation to the severity of lesions in the coronary arteries. Pol Arch Med Wewn. 2007;117:343–9. [PubMed] [Google Scholar]
- 34.Stefanadi E, Tousoulis D, Antoniades C, et al. Early initiation of low-dose atorvastatin treatment after an acute ST-elevated myocardial infarction, decreases inflammatory process and prevents endothelial injury and activation. Int J Cardiol. 2009;133:266–8. doi: 10.1016/j.ijcard.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 35.Sorescu D, Griendling KK. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail. 2002;8:132–40. doi: 10.1111/j.1527-5299.2002.00717.x. [DOI] [PubMed] [Google Scholar]
- 36.Downey JM, Omar B, Ooiwa H, McCord J. Superoxide dismutase therapy for myocardial ischemia. Free Radic Res Commun. 1991;12–13:703–20. doi: 10.3109/10715769109145850. [DOI] [PubMed] [Google Scholar]
- 37.Pritchard KA, Jr, Groszek L, Smalley DM, et al. Native low-density lipoprotein increases endothelial cell nitric oxide synthase generation of superoxide anion. Circ Res. 1995;77:510–8. doi: 10.1161/01.res.77.3.510. [DOI] [PubMed] [Google Scholar]
- 38.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-kappaB: Its role in health and disease. J Mol Med. 2004;82:434–48. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 39.Goodwin BL, Pendleton LC, Levy MM, Solomonson LP, Eichler DC. Tumor necrosis factor-alpha reduces argininosuccinate synthase expression and nitric oxide production in aortic endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H1115–21. doi: 10.1152/ajpheart.01100.2006. [DOI] [PubMed] [Google Scholar]
- 40.Gao X, Belmadani S, Picchi A, et al. Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation. 2007;115:245–54. doi: 10.1161/CIRCULATIONAHA.106.650671. [DOI] [PubMed] [Google Scholar]
- 41.Steinberg D. Antioxidants in the prevention of human atherosclerosis. Circulation. 1992;85:2338–44. doi: 10.1161/01.cir.85.6.2337. [DOI] [PubMed] [Google Scholar]
- 42.Prasad K, Lee P. Suppression of hypercholesterolemic atherosclerosis by pentoxifylline and its mechanism. Atherosclerosis. 2007;192:313–22. doi: 10.1016/j.atherosclerosis.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 43.Cooke JP, Singer AH, Tsao P, Zera P, Rowan RA, Billingham ME. Antiatherogenic effects of L-arginine in the hypercholesterolemic rabbit. J Clin Invest. 1992;90:1168–72. doi: 10.1172/JCI115937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peter K, Nawroth P, Conradt C, et al. Circulating vascular cell adhesion molecule-1 correlates with the extent of human atherosclerosis in contrast to circulating intercellular adhesion molecule-1, E-selectin, P-selectin, and thrombomodulin. Arterioscler Thromb Vasc Biol. 1997;17:505–12. doi: 10.1161/01.atv.17.3.505. [DOI] [PubMed] [Google Scholar]