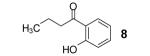
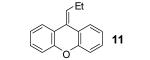
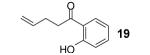
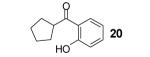
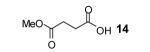
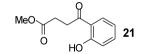
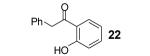
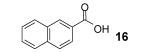
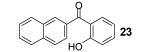
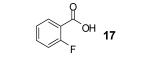
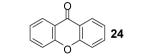
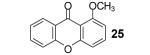
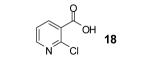
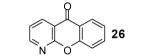
Table 1.
Reaction of Carboxylic Acids with Arynesa
Reaction conditions: 0.25 mmol of acid, 1.5 equiv of benzyne precursor and 4.0 equiv of CsF in 15 mL of THF were heated in a closed vial at 125 °C for 24 h.
Isolated yield.
3.0 Equiv of benzyne precursor, 6.0 equiv of CsF and 5 mL of DME were used.
3-Methoxy-2-(trimethylsilyl)phenyl triflate was used as the aryne precursor.