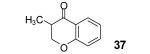
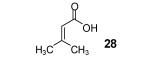
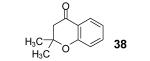
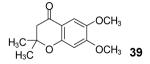
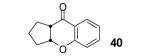
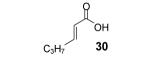
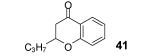
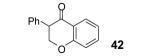
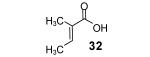
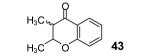
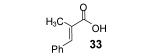
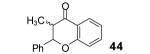
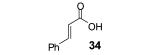
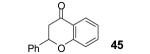
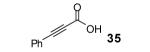
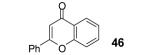
Table 2.
Reaction of Acrylic and Propiolic Acids with Arynesa
Reaction conditions: 0.25 mmol of acid, 1.5 equiv of aryne precursor and 4.0 equiv of CsF in 15 mL of THF were heated in a closed vial at 125 °C for 18 h. Then an additional 0.5 equiv of aryne precursor and 1.0 equiv of CsF was added and the heating was continued at 125 °C for 6 h.
Isolated yield.
4,5-Dimethoxy-2-(trimethylsilyl)phenyl triflate was used as the aryne precursor.
The E/Z ratio is ~1.8/1.
The E/Z ratio is ~5.1/1.
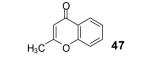
The yield also includes the product obtained after base-induced cyclization of the o-hydroxyaryl ketone (see the Supporting Information).
Reaction conditions: 0.25 mmol of acid, 1.5 equiv of aryne precursor and 2.0 equiv of TBAT in 5 mL of toluene were heated at 60 °C for 24 h.