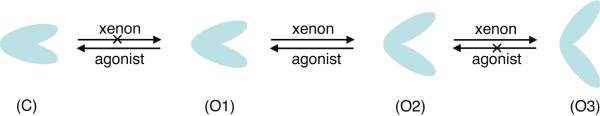
Figure 7.
A diagram showing how xenon affects the domain closure of the ligand-binding domain (LBD) of the NMDA receptor. Multiple conformations exist with various degrees of domain closure, such as C, O1, O2 and O3. Agonist binding initiates the S1S2 cleft closing (C) and the channel opening. Xenon may weaken the agonist binding, but is unable to replace the agonist and open the cleft. Xenon can also inhibit the channel in a non-competitive manner by altering the interaction between NR1 and NR2 (not shown here). For LBD without agonists, xenon may promote the cleft opening from O1 to O2 or from O2 to O3. When the S1S2 cleft is widely opened (O3) by xenon, the separation between two domains is too large to allow agonist binding at the hinge region of the S1S2 cleft. This is another format of non-competitive xenon inhibition. The competitive inhibition can occur between two intermediate states O1 and O2: xenon promotes the transition from O1 to O2, while agonists work in the opposite direction.