Abstract
Purpose
Positive surgical margins (PSM) in men undergoing radical prostatectomy (RP) for prostate cancer (PCa) are associated with an increased risk of biochemical recurrence. Little data have evaluated the role of PSM in PCa-specific mortality (PCSM). Using a large, population-based national cancer registry, we evaluate the risk of PCSM associated with margin status.
Methods
The SEER cancer registry data for patients diagnosed in 1998–2006 were used to identify men undergoing RP for PCa. Margin status, pathologic stage, Gleason grade and post-operative radiation therapy were recorded along with demographic data. Multivariate Cox regression analysis was used to estimate the risk of PCSM associated with PSMs.
Results
A total of 65,633 patients comprised the cohort in which 291 (0.44%) PCa-specific deaths occurred over an average follow-up of 50 months. PSMs were reported in 21.2% and were more common in pT3a than pT2 tumors (44% vs. 18%, p<0.001) and higher grade tumors (28% vs. 18%, p<0.001). The 7-year disease-specific survival rates for those at highest risk of PCSM (higher grade pT3a) were 97.3% for cases with negative surgical margins and 92.4% for those with PSMs. PSMs were associated with a 2.9-fold increased risk of PCSM (HR 2.55, 95% CI 2.02 – 3.21). PSM remained an independent predictor of PCSM in the multivariate analysis (HR 1.70, 95% CI 1.32 – 2.18).
Conclusion
These data demonstrate the independent role of positive surgical margin in PCSM. These finding support the importance of optimizing surgical technique to achieve a sound oncologic surgical outcome with negative surgical margins when possible.
Keywords: prostate cancer, surgical margin, survival, population-based, radical prostatectomy
Introduction
A number of nomograms exist for predicting outcomes after radical prostatectomy (RP) for prostate cancer patients (PCa).1-6 Several clinical and pathologic factors have been included in these models, most of which cannot be altered by the treating physician (e.g., pathologic stage, Gleason score, pre-treatment PSA and age). There are also various nomograms available to predict the probability of extracapsular extension and thus guide the surgeon to consider “wide field” cavernosal nerve resection versus a nerve-sparing in an attempt to reduce positive surgical margins (PSMs).7, 8
Most studies have found PSMs to be an independent predictor of biochemical recurrence (BCR) after RP.9-14 However, BCR represents an early event in the natural history of PCa with heterogeneous outcomes, and BCR has not been accepted as an accurate surrogate for PCa-specific mortality (PCSM), a more informative end-point.15, 16 For example, worse PCSM has only been shown for men with BCR and a short PSA doubling time (PSADT) or rapid time to BCR from primary treatment.17, 18
Small studies have evaluated the relation between PSM and biopsy proven local recurrence12 and PCSM14 after RP, but these have been limited by few events (n < 10) and the potential for referral bias. In addition, because of the low case-fatality rate from PCa, it may be that surgical margins do not appreciably add to the more established pathological factors that impact PCSM. Only a large study with enough events can evaluate whether PSM is an independent predictor of PCSM. In this study, using a national population-based tumor registry with margin status data, we investigate the risk of PCSM due to PSMs.
Methods
Data Source
The Surveillance, Epidemiology, and End Results (SEER) Program database was used to identify the cohort of patients for this study. SEER collects cancer incidence, primary treatment and survival data from 17 population-based cancer registries accounting for approximately 26% of the United States population.19 Data from 1998 through 2006 from 13 SEER registries were used (metropolitan areas of San Francisco-Oakland, San Jose-Monterey, Los Angeles, Atlanta, Detroit, Seattle-Puget Sound and the states of Connecticut, Hawaii, New Mexico, Utah, Iowa). Cases prior to 1998 were excluded since margin status was not reported prior to that date. The registries from Greater California, Kentucky, Louisiana and New Jersey were excluded since they did not report to SEER for the entire study period having joined in 2000. The Alaska and Rural Georgia registries were also excluded since they provided less the 0.4% of the total cases.
Study Population
Potential subjects were identified using the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) site codes for the prostate (C61.9) and ICD-O-3 histology codes for adenocarcinoma (8550) and acinar cell carcinoma (8140). Only cases treated with RP were included. There were 71,509 eligible cases. Cases with missing tumor grade (n = 333, 0.5%) or stage (n = 1,835, 2.5%) were excluded. Margin status is not reported for pathologic stage pT3b (seminal vesicle invasion) or pT4 (adjacent organ invasion). We also excluded patients with node positive disease (n = 830, 1.3%). There were 3,181 (4.4%) pT3b and 1,088 (1.5%) pT4 cases that were excluded. One hundred (0.14%) cases received radiation therapy (XRT) prior to RP and were excluded.
Data Collection and Coding
Margin status and pathologic T-stage was determined from the site-specific staging codes for prostate. The SEER grading system was used since specific Gleason grades were not recorded prior to 2004. The SEER grading system uses “Well Differentiated,” “Moderately Differentiated,” and Poorly Differentiated,” which correspond to Gleason scores “2 –4”, “5 – 7”, and “8 – 10” respectively. Gleason score 7 was moved from “Moderately Differentiated” to “Poorly Differentiated” with cases diagnosed after January 1, 2003. Only 1.5% of cases were graded as “Well Differentiated” during the study period. For this analysis, “Well Differentiated” and “Moderately Differentiated” were combined into a “Lower Grade” category with the remaining cases considered “Higher Grade.” The XRT variable in SEER does not distinguish between adjuvant and salvage radiation therapy. Race was categorized as Caucasian, African-American or Other. Age was categorized in 5-year age groups (< 55, 55 – 59, 60 – 64, 65 – 69, ≥ 70).
Statistical Analysis
We used univariate statistics to compare demographic and pathological characteristics between subjects with and without PSMs. Kaplan-Meier curves were used to plot time to PCSM by margin status. Multivariate Cox regression was performed to evaluate the risk of PCSM associated with margin status. Covariates selected for inclusion in the final model based on an a priori relationship with PCa-survival included stage, grade, additional XRT, age and race. In addition, tumor regristry and year of diagnosis were included in the multivariate model. Robust standard errors were used. Because of the strong effect of pathologic stage and grade on outcomes after RP and their relationship with margin status, we performed stratified analyses based on stage (pT2 vs. pT3a); grade (lower grade vs. higher grade); and combinations of grade and stage (pT2 lower grade; pT2 higher grade; pT3a lower grade, pT3a higher grade). The same covariates were included as in the full model except for the stratified variable(s). Potential interaction between margin status and stage/grade were evaluated with the likelihood ratio test. Hazard ratios are presented along with their 95% confidence intervals. All statistical analyses were conducted using Stata® software version 8.
Results
A total of 65,633patients with PCa underwent RP and were available for analysis. PSMs were recorded in 21.2% of cases overall. The median follow-up for the cohort was 50 months (range 1 – 107 months). A total of 2,927 (4.5%) cases died of non-PCa related causes and 291 (0.5%) died due to PCa. The cumulative PCSM over the study period was greater in those with positive margins than in those with negative margins (0.86% vs 0.33%, p < 0.001) while the non-PCa cumulative mortality was similar between positive and negative margin cases (4.5% and 4.3% respectively, p=0.42).
Table 1 compares the clinical and pathologic characteristics between those with and without PSMs. Margin positivity varied substantially across registries (11.3% to 28.5%). The annual PSM frequency ranged between 17.9% to 23.5%, although it declined steadily for the last 5 years of the study period (p < 0.001). As expected, a PSM was more common in patients with pT3 disease (43.8% vs. 17.7%, p < 0.001) and higher grade disease (275% vs. 18.3%, p < 0.001). Receiving post-RP radiation therapy (data not shown) was rare overall (3.4%) and given more frequently to patients with PSMs (10.2%) than to those without PSMs (1.5%, p < 0.001). XRT was also more common in those with pT3a disease. The prevalence of XRT for pT2 with negative surgical margins (NSM), pT2 with PSM, pT3a with NSM and pT3a with PSM was < 1.0%, 6.7%, 7.7% and 19.2%, respectively, p < 0.001).
Table 1. Distribution of Clinical and Pathological Characteristics of 65,633 Radical Prostatectomy Patients by Surgical Margin Status.
| Negative Margins N (%) | Positive Margins N (%) | P-value | |
|---|---|---|---|
| Overall | 51,728 (78.8) | 13,905 (21.2) | |
| Age (years) | |||
| < 55 | 9,699 (79.3) | 2,533 (20.7) | 0.27 |
| 55 – 59 | 11,499 (79.1) | 3,036 (20.9) | |
| 60 – 64 | 12,497 (78.8) | 3,360 (21.2) | |
| 65 – 69 | 11,084 (78.3) | 3,071 (21.7) | |
| 70 + | 6,947 (78.5) | 1,904 (21.5) | |
| Race | |||
| Caucasian | 43,216 (78.3) | 11,313 (20.8) | < 0.001 |
| African-American | 5,359 (75.3) | 1,754 (24.7) | |
| Other | 3,153 (79.0) | 838 (21.0) | |
| Tumor Registry | |||
| San Francisco-Oakland | 4,988 (82.4) | 1,066 (17.6) | < 0.001 |
| Connecticut | 4,744 (81.4) | 1,084 (18.6) | |
| Detroit (Metro) | 7,341 (79.9) | 1,843 (20.1) | |
| Hawaii | 1,039 (79.3) | 271 (21.7) | |
| Iowa | 4,341 (78.1) | 1,221 (22.0) | |
| New Mexico | 2,427 (84.3) | 453 (15.7) | |
| Seattle/Puget Sound | 6,296 (77.6) | 1,821 (22.4) | |
| Utah | 3,083 (71.5) | 1,231 (28.5) | |
| Atlanta (Metro) | 3,194 (88.7) | 407 (11.3) | |
| San Jose-Monterey | 2,542 (75.9) | 786 (23.6) | |
| Los Angeles | 11,733 (75.9) | 3,722 (24.1) | |
| Year of Diagnosis | |||
| 1998 | 4,987 (79.8) | 1,261 (20.2) | < 0.001 |
| 1999 | 5,569 (78.4) | 1,536 (21.6) | |
| 2000 | 5,545 (78.3) | 1,540 (21.7) | |
| 2001 | 5,854 (78.1) | 1,643 (21.9) | |
| 2002 | 5,884 (76.5) | 1,812 (23.5) | |
| 2003 | 5,710 (77.7) | 1,632 (22.2) | |
| 2004 | 6,240 (78.4) | 1,715 (21.6) | |
| 2005 | 5,716 (80.3) | 1,407 (19.8) | |
| 2006 | 6,223 (82.1) | 1,359 (17.9) | |
| Pathologic Stage | |||
| pT2 | 46,818 (82.3) | 10,074 (17.7) | < 0.001 |
| pT3a | 4,910 (56.2) | 3,831 (43.8) | |
| Grade | |||
| Lower Grade | 36,786 (81.7) | 8,233 (18.3) | < 0.001 |
| Higher Grade | 14,942 (72.5) | 5,672 (27.5) |
Table 2 lists the 5- and 7-year disease-specific survival (DSS) rates (along with 95% CI) for men by margin status stratified by grade and stage. For lower grade, pT2 tumors, the disease-specific survival is essentially identical for PSM and NSM (99.7% and 99.8%, respectively). The 5- and 7-yr disease-specific survival for higher grade pT2 and lower grade pT3a tumors are slightly higher for men with NSMs versus PSMs, although there is some overlap in the 95% CIs. For higher grade pT3a tumors, the 7-yr disease-specific survival is greater for those with NSMs than PSMs (97.3% vs. 92.4%, respectively (p < 0.001)).
Table 2. 5- and 7-year Prostate Cancer-Specific Survival Based on Surgical Margin Status in Men Undergoing Radical Prostatectomy Stratified by Stage and Grade.
| 5-year DSS (95% CI) |
7-year DSS (95% CI) |
|||
|---|---|---|---|---|
| Negative Margin | Positive Margin | Negative Margin | Positive Margin | |
| pT2 | ||||
| Lower Grade | 99.8 (99.7 – 99.8) |
99.7 (99.5 – 99.8) |
99.6 (99.5 – 99.7) |
99.4 (99.0 – 99.6) |
| Higher Grade | 99.4 (99.1 – 99.6) |
99.0 (98.2 – 99.4) |
98.6 (98.1 – 99.0) |
97.3 (95.5 – 98.4) |
| pT3a | ||||
| Lower Grade | 99.7 (99.3 – 99.8) |
99.2 (98.5 – 99.6) |
99.4 (98.8 – 99.7) |
98.9 (97.9 – 99.4) |
| Higher Grade | 98.3 (97.1 – 99.0) |
96.5 (95.1 – 97.5) |
97.6 (96.0 – 98.6) |
92.4 (89.7 – 94.5) |
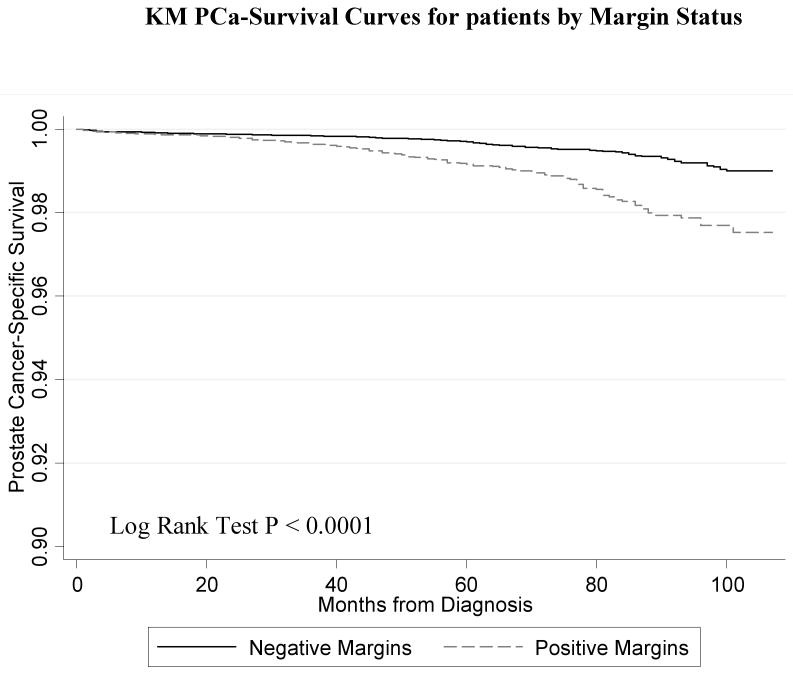
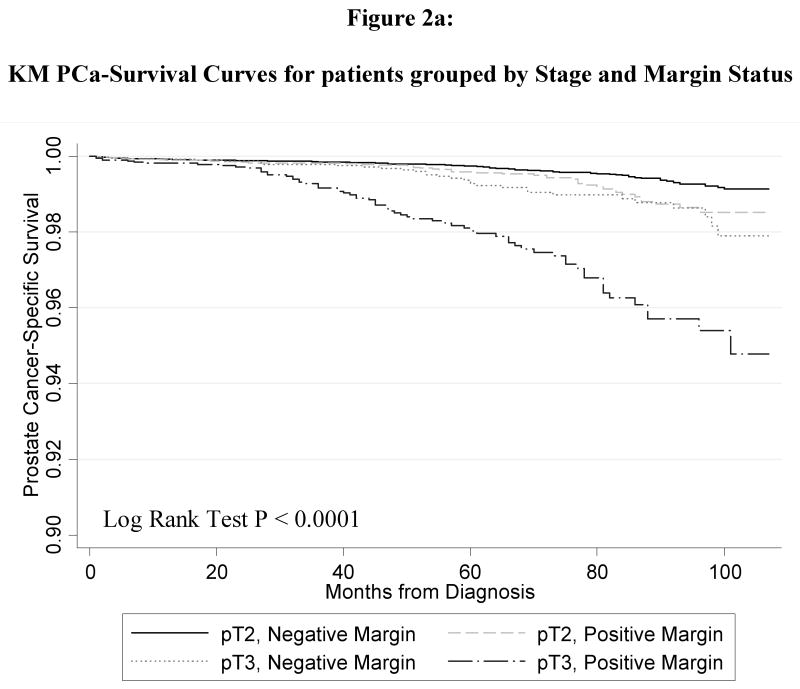
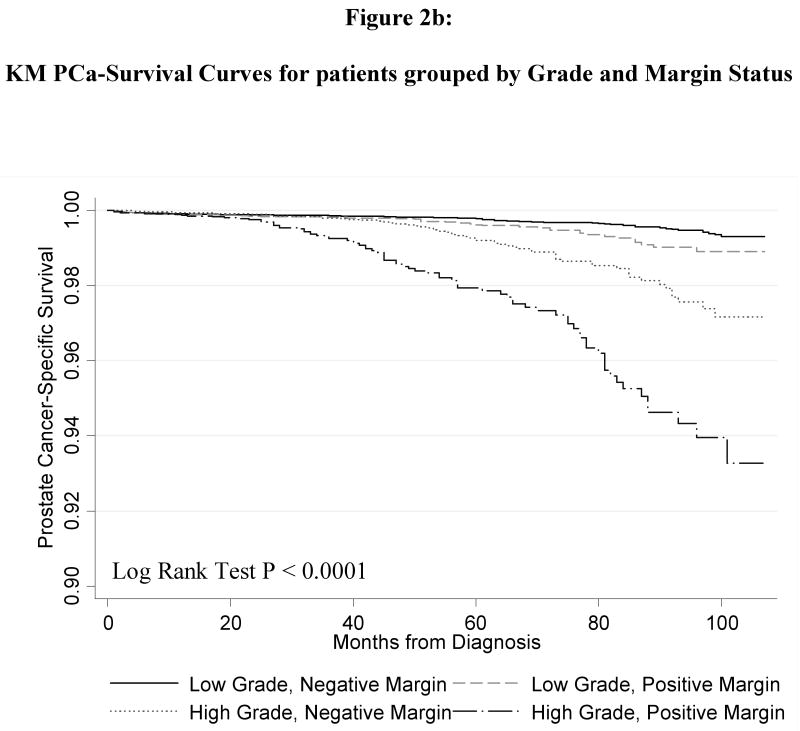
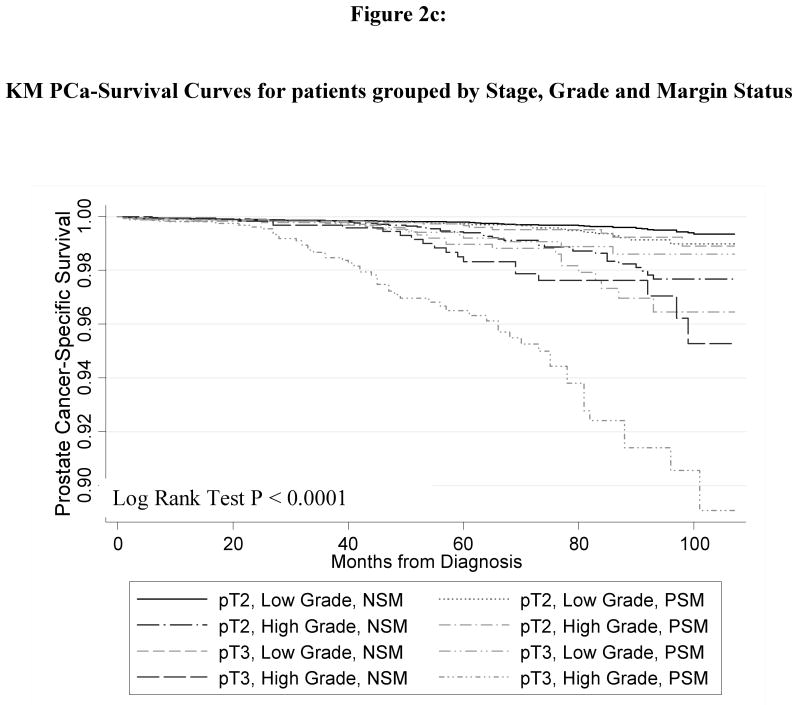
Figure 1 shows the Kaplan-Meier curve for the PCSM in men with and without PSMs. Men with PSMs had significantly greater PCSM (p < 0.0001) corresponding to a2.6-fold increased risk of PCSM in the univariate Cox regression model (HR 2.55, 95% CI 2.02 – 3.21). Figure 2 shows the PCSM Kaplan-Meier curves, stratified by stage and margin status (Figure 2A); grade and margin status (Figure 2B); and grade, stage and margin status (Figure 2C). In Table 3, the results of the multivariate model are shown. PSM remained an independent predictor of PCSM, with a 70% increased risk (HR 1.70, 95% CI 1.32 – 2.18). Higher grade disease, pT3 disease and additional XRT were also predictive of PCSM in the multivariate model
Figure 1.
Kaplan Meier prostate-cancer specific survival plots stratified by surgical margin status (p < 0.001).
Figure 2.
Kaplan Meier prostate-cancer specific survival plots stratified by (a) pathologic stage and surgical margin status; (b) pathologic grade and surgical margin status; (c) pathologic stage, grade and surgical margin status. All p-value < 0.001.
Table 3. Risk of PCa-Specific Mortality in Men Undergoing Radical Prostatectomy.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR | 95% CI | HR * | 95% CI | |
| Positive Margin | 2.55 | 2.02 – 3.21 | 1.70 | 1.32 – 2.18 |
| Grade | ||||
| Low Grade | 1.00 | Referent | 1.00 | Referent |
| High Grade | 2.05 | 1.80 – 2.32 | 3.45 | 2.69 – 4.42 |
| Additional XRT | 4.04 | 2.89 – 5.66 | 1.77 | 1.20 – 2.61 |
| Stage | ||||
| pT2 | 1.00 | Referent | 1.00 | Referent |
| pT3 | 3.43 | 2.69 – 4.36 | 2.01 | 1.55 – 2.60 |
Adjusted for all other covariates in the table along with age, race, registry and year of diagnosis
In Table 4, the multivariate models (adjusting for the same variables in the full model) are stratified by stage and grade. The HRs for PCSM are presented within each strata as there was evidence for effect modification by the likelihood ratio test for an interaction between margin status with stage (p = 0.04), and margin status with grade (p = 0.12). In each case, PSM is associated with an increased risk of PCSM, although it only reached statistical significance in those with higher grade tumors (HR for PSM: 1.97, 95% CI 1.41 – 2.76); pT3a tumors (HR for PSM: 2.42, 95% CI 1.58 – 3.72) and for those tumors that are both pT3a and higher grade (HR for PSM: 2.72, 95% CI 1.62 – 4.54).
Table 4. Adjusted Risk of Prostate Cancer-Specific Mortality in Men undergoing Radical Prostatectomy, Stratified by Stage and Grade.
| N (%) | HR * | 95% CI | |
|---|---|---|---|
| Grade | |||
| Lower Grade | |||
| Negative Margin | 36,786 (81.7) | 1.00 | Referent |
| Positive Margin | 8,233 (18.3) | 1.36 | 0.91 – 2.04 |
| Higher Grade | |||
| Negative Margin | 14,942 (72.5) | 1.00 | Referent |
| Positive Margin | 5,672 (27.5) | 1.97 | 1.41 – 2.76 |
| Pathologic Stage | |||
| pT2 | |||
| Negative Margin | 46,818 (82.3) | 1.00 | Referent |
| Positive Margin | 10,074 (17.7) | 1.29 | 0.90 – 1.84 |
| pT3a | |||
| Negative Margin | 4,910 (56.2) | 1.00 | Referent |
| Positive Margin | 3,831 (43.8) | 2.42 | 1.58 – 3.72 |
| Stage and Grade | |||
| pT2 | |||
| Lower Grade | |||
| Negative Margin | 34,309 (83.9) | 1.00 | Referent |
| Positive Margin | 6,575 (16.1) | 1.21 | 0.75 – 1.95 |
| Higher Grade | |||
| Negative Margin | 12,509 (78.1) | 1.00 | Referent |
| Positive Margin | 3,499 (21.9) | 1.38 | 0.80 – 2.38 |
| pT3a | |||
| Lower Grade | |||
| Negative Margin | 2,477 (59.9) | 1.00 | Referent |
| Positive Margin | 1,658 (40.1) | 1.84 | 0.79 – 4.27 |
| Higher Grade | |||
| Negative Margin | 2,433 (52.8) | 1.00 | Referent |
| Positive Margin | 2,173 (47.2) | 2.72 | 1.62 – 4.54 |
Adjusted for stage and grade when not the stratified variable. Also adjusted for xrt, age, race, registry and year of diagnosis
Discussion
Although PSMs have been shown to be associated with BCR after RP, studies showing its significance in disease-specific mortality have not been reported. In this large, population-based study of over 65,000 RP patients, we report the independent predictive role of surgical margin status on men undergoing RP. Our findings support the importance of achieving negative surgical margins when possible, especially in those with higher grade disease and suspicion of extracapsular extension who are at greatest risk of early PCSM.
Multiple studies have found PSMs to be associated with higher rates of BCR after RP.9-14 However, many patients experiencing BCR will not die from PCa and consequently BCR is not a universally accepted surrogate for PCSM.15, 16 Studies evaluating margin status in relation to the more clinically robust endpoint of PCSM have been lacking. Recently, German investigators reported a series of 406 patients undergoing RP with PSM seen in 18%.14 PSMs were associated in multivariate analysis with BCR (114 patients, HR 3.2, 95% CI 2.1 – 4.9), local recurrence (22 patients, HR 4.6, 95% CI 1.8 – 12.1) and distant metastasis (HR 16 patients, HR 6.65, 95% CI 1.9 – 23.1). Although there were too few events to evaluate the impact of PSMs on PCSM in multivariate analysis, in the univariate analysis, PCSM was more common in those with PSMs compared to NSMs (8.6% vs. 0.6%, p < 0.001). In our study, we found in multivariate analysis that PSMs remains an independent predictor of PCSM, with a 70% increased risk of death due to PCa compared to patients with NSMs.
In our study, PSMs were associated with a increased risk of PCSM within each strata of stage and grade, although the HR only reached statistical significance in those with higher grade tumors (HR for PSM: 1.97, 95% CI 1.41 – 2.76) or pT3a disease (HR for PSM: 2.42, 95% CI 1.68 – 3.72) or both pT3a and high grade tumors (HR for PSM: 2.72, 95% CI 1.62 – 4.54). It is possible that with increased follow-up and more events, margin status may reach statistical significance in pT2 and lower grade tumors. However, due to the low disease-specific death rate for these patients, surgical margin status may only become a significant contributor to PCSM in those with a life expectancy > 10 years for whom the risk of PCSM is higher. In contrast, when looking at those at highest risk of PCSM (those with pT3a higher grade disease), the 7-year PCa-specific survival rates are 97.6% and 92.4% for NSMs and PSMs, respectively. This absolute 5.0% difference is similar to the difference reported in the Scandinavian randomized trial between surgery and watchful waiting.20 Our findings indicate that surgical technique, not just surgery alone, appears to be associated with improved disease-specific survival.
Intra-operatively, PSMs can occur due to extensive cancer for which complete resection is impossible/unadvisable; or due to technical error (e.g, capsular incision). Pathological interpretation of margin status is complicated by surgical artifact (crush, tears, thermal or electrocautery) causing unpredictable tracking of ink, which can lead to variability in reporting of surgical margins even for expert urologic pathologists.21 Additionally, the pathological processing of the prostate can introduce variance in margin detection due to differences in tissue handling and processing. Differences in sampling prostatectomy specimens can result in higher false negative rates at those institutions where sampling is selective rather than comprehensive, viz. where the prostate is totally embedded and sectioned.22 Sampling methods that are biased toward the peripheral zone of the prostate, the zone in which the majority of cancers occur, undersample the transition zone, in which at least 15 % of prostate adenocarcinomas are located.23 A potential consequence of not sampling the anterior aspect of the prostate is missing margin-positive areas in this zone. Future efforts to minimize these effects to reduce ‘false’ positive (or negative) margins are needed to help better standardize margin status and its impact on disease outcomes. The importance of accurate margin status has been demonstrated in an analysis of EORTC trial 22911 of adjuvant XRT after prostatectomy.24 Patients reported to have PSM after review of the prostate specimen by a pathologist with urologic oncology expertise was the strongest predictor of benefit from adjuvant radiation therapy in this randomized trial. The multivariate association of XRT with worse PCSM in our study should be interpreted with caution as we are unable to distinguish between adjuvant or salvage XRT thus selection bias may play a role in this finding.
This study has limitations. We do not have PSA data or information on the precise pathological factors such as the presence of lymphovascular invasion, number of PSMs and location of PSMs which have been reported to affect BCR.11, 25, 26 In addition, we do not have central pathologic review, which could lead to misclassification. However, the reported rates of PSMs from the different tumor registries in our series (11% – 29%) are similar to those reported in a recent review that found PSM rates of 11% – 38%.27 Further, the association between common pathologic criteria and margin status (higher PSMs with higher stage and higher Gleason score) support the reliability of abstracted margin status by the SEER registries. Finally, we do not have data on whether or not the RP was nerve sparing. Despite these limitations, this large population-based series show a difference in PCSM associated with PSMs. These data demonstrate the importance of optimizing surgical technique to achieve a negative surgical margin in PCa and underscore the need for pathologic standardizations of tissue processing to accurately define surgical margin status.
Acknowledgments
T32 CA009168-30; with additional support from the Fred Hutchinson Cancer Research Center.
References
- 1.Cooperberg MR, Pasta DJ, Elkin EP, Litwin MS, Latini DM, Du Chane J, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173(6):1938–42. doi: 10.1097/01.ju.0000158155.33890.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eggener SE, Vickers AJ, Serio AM, Donovan MJ, Khan FM, Bayer-Zubek V, et al. Comparison of models to predict clinical failure after radical prostatectomy. Cancer. 2009;115(2):303–10. doi: 10.1002/cncr.24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedland SJ, Amling CL, Dorey F, Kane CJ, Presti JC, Jr, Terris MK, et al. Race as an outcome predictor after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Urology. 2002;60(4):670–4. doi: 10.1016/s0090-4295(02)01847-2. [DOI] [PubMed] [Google Scholar]
- 4.Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ, Jr, Dotan ZA, DiBlasio CJ, et al. Postoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2005;23(28):7005–12. doi: 10.1200/JCO.2005.01.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suardi N, Porter CR, Reuther AM, Walz J, Kodama K, Gibbons RP, et al. A nomogram predicting long-term biochemical recurrence after radical prostatectomy. Cancer. 2008;112(6):1254–63. doi: 10.1002/cncr.23293. [DOI] [PubMed] [Google Scholar]
- 6.Walz J, Chun FK, Klein EA, Reuther A, Saad F, Graefen M, et al. Nomogram predicting the probability of early recurrence after radical prostatectomy for prostate cancer. J Urol. 2009;181(2):601–7. doi: 10.1016/j.juro.2008.10.033. discussion 07-8. [DOI] [PubMed] [Google Scholar]
- 7.Makarov DV, Trock BJ, Humphreys EB, Mangold LA, Walsh PC, Epstein JI, et al. Updated nomogram to predict pathologic stage of prostate cancer given prostate-specific antigen level, clinical stage, and biopsy Gleason score (Partin tables) based on cases from 2000 to 2005. Urology. 2007;69(6):1095–101. doi: 10.1016/j.urology.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohori M, Kattan MW, Koh H, Maru N, Slawin KM, Shariat S, et al. Predicting the presence and side of extracapsular extension: a nomogram for staging prostate cancer. J Urol. 2004;171(5):1844–9. doi: 10.1097/01.ju.0000121693.05077.3d. discussion 49. [DOI] [PubMed] [Google Scholar]
- 9.Simon MA, Kim S, Soloway MS. Prostate specific antigen recurrence rates are low after radical retropubic prostatectomy and positive margins. J Urol. 2006;175(1):140–4. doi: 10.1016/S0022-5347(05)00050-9. discussion 44-5. [DOI] [PubMed] [Google Scholar]
- 10.Karakiewicz PI, Eastham JA, Graefen M, Cagiannos I, Stricker PD, Klein E, et al. Prognostic impact of positive surgical margins in surgically treated prostate cancer: multi-institutional assessment of 5831 patients. Urology. 2005;66(6):1245–50. doi: 10.1016/j.urology.2005.06.108. [DOI] [PubMed] [Google Scholar]
- 11.Pettus JA, Weight CJ, Thompson CJ, Middleton RG, Stephenson RA. Biochemical failure in men following radical retropubic prostatectomy: impact of surgical margin status and location. J Urol. 2004;172(1):129–32. doi: 10.1097/01.ju.0000132160.68779.96. [DOI] [PubMed] [Google Scholar]
- 12.Swindle P, Eastham JA, Ohori M, Kattan MW, Wheeler T, Maru N, et al. Do margins matter? The prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol. 2005;174(3):903–7. doi: 10.1097/01.ju.0000169475.00949.78. [DOI] [PubMed] [Google Scholar]
- 13.Ward JF, Zincke H, Bergstralh EJ, Slezak JM, Myers RP, Blute ML. The impact of surgical approach (nerve bundle preservation versus wide local excision) on surgical margins and biochemical recurrence following radical prostatectomy. J Urol. 2004;172(4 Pt 1):1328–32. doi: 10.1097/01.ju.0000138681.64035.dc. [DOI] [PubMed] [Google Scholar]
- 14.Pfitzenmaier J, Pahernik S, Tremmel T, Haferkamp A, Buse S, Hohenfellner M. Positive surgical margins after radical prostatectomy: do they have an impact on biochemical or clinical progression? BJU Int. 2008;102(10):1413–8. doi: 10.1111/j.1464-410X.2008.07791.x. [DOI] [PubMed] [Google Scholar]
- 15.Collette L. Prostate-specific antigen (PSA) as a surrogate end point for survival in prostate cancer clinical trials. Eur Urol. 2008;53(1):6–9. doi: 10.1016/j.eururo.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 16.Stephenson AJ, Kattan MW, Eastham JA, Dotan ZA, Bianco FJ, Jr, Lilja H, et al. Defining biochemical recurrence of prostate cancer after radical prostatectomy: a proposal for a standardized definition. J Clin Oncol. 2006;24(24):3973–8. doi: 10.1200/JCO.2005.04.0756. [DOI] [PubMed] [Google Scholar]
- 17.D'Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen MH. Surrogate end point for prostate cancer specific mortality in patients with nonmetastatic hormone refractory prostate cancer. J Urol. 2005;173(5):1572–6. doi: 10.1097/01.ju.0000157569.59229.72. [DOI] [PubMed] [Google Scholar]
- 18.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. Jama. 2005;294(4):433–9. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; Surveillance, Epidemiology, and End Results (SEER) Program Populations (1969-2005) www.seer.cancer.gov/popdata. [Google Scholar]
- 20.Bill-Axelson A, Holmberg L, Ruutu M, Haggman M, Andersson SO, Bratell S, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352(19):1977–84. doi: 10.1056/NEJMoa043739. [DOI] [PubMed] [Google Scholar]
- 21.Evans AJ, Henry PC, Van der Kwast TH, Tkachuk DC, Watson K, Lockwood GA, et al. Interobserver variability between expert urologic pathologists for extraprostatic extension and surgical margin status in radical prostatectomy specimens. Am J Surg Pathol. 2008;32(10):1503–12. doi: 10.1097/PAS.0b013e31817fb3a0. [DOI] [PubMed] [Google Scholar]
- 22.Sehdev AE, Pan CC, Epstein JI. Comparative analysis of sampling methods for grossing radical prostatectomy specimens performed for nonpalpable (stage T1c) prostatic adenocarcinoma. Hum Pathol. 2001;32(5):494–9. doi: 10.1053/hupa.2001.24322. [DOI] [PubMed] [Google Scholar]
- 23.Garcia JJ, Al-Ahmadie HA, Gopalan A, Tickoo SK, Scardino PT, Reuter VE, et al. Do prostatic transition zone tumors have a distinct morphology? Am J Surg Pathol. 2008;32(11):1709–14. doi: 10.1097/PAS.0b013e318172ee97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van der Kwast TH, Bolla M, Van Poppel H, Van Cangh P, Vekemans K, Da Pozzo L, et al. Identification of patients with prostate cancer who benefit from immediate postoperative radiotherapy: EORTC 22911. J Clin Oncol. 2007;25(27):4178–86. doi: 10.1200/JCO.2006.10.4067. [DOI] [PubMed] [Google Scholar]
- 25.Eastham JA, Kuroiwa K, Ohori M, Serio AM, Gorbonos A, Maru N, et al. Prognostic significance of location of positive margins in radical prostatectomy specimens. Urology. 2007;70(5):965–9. doi: 10.1016/j.urology.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 26.Obek C, Sadek S, Lai S, Civantos F, Rubinowicz D, Soloway MS. Positive surgical margins with radical retropubic prostatectomy: anatomic site-specific pathologic analysis and impact on prognosis. Urology. 1999;54(4):682–8. doi: 10.1016/s0090-4295(99)00204-6. [DOI] [PubMed] [Google Scholar]
- 27.Yossepowitch O, Bjartell A, Eastham JA, Graefen M, Guillonneau BD, Karakiewicz PI, et al. Positive Surgical Margins in Radical Prostatectomy: Outlining the Problem and Its Long-Term Consequences. Eur Urol. 2008 doi: 10.1016/j.eururo.2008.09.051. [DOI] [PubMed] [Google Scholar]