FIG. 2.
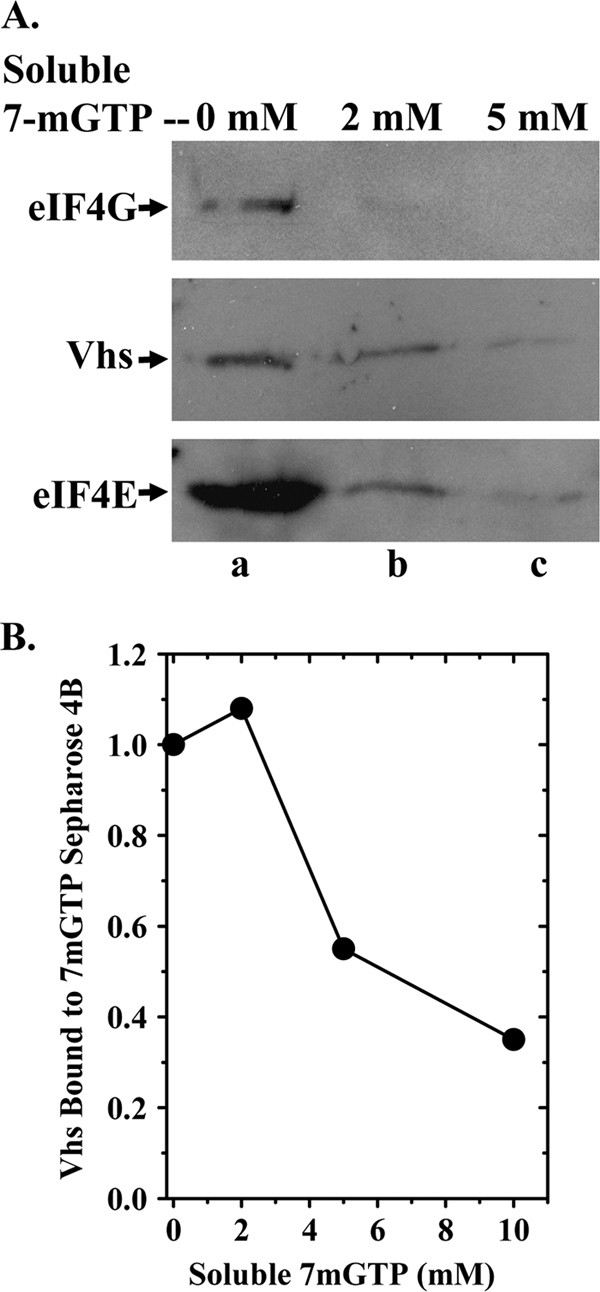
Soluble 7-methyl GTP competes with 7-methyl GTP-Sepharose 4B for binding eIF4F and associated Vhs. (A) Cytoplasmic extracts were prepared from HeLa cells 10 h after infection with 10 PFU/cell of wild-type HSV-1 (strain KOS) and incubated for 1 h with 7-methyl GTP-Sepharose 4B as described for Fig. 1, except that the reaction mixtures contained 0 mM, 2 mM, or 5 mM 7-methyl GTP as indicated above the lanes. Bound proteins were eluted by boiling in SDS sample buffer and analyzed by SDS-PAGE and Western blotting for Vhs, eIF4E, or eIF4G, as indicated to the left of lane a. (B) [35S]methionine-labeled Vhs was produced by coupled in vitro transcription and translation and assayed for binding to 7-methyl GTP-Sepharose 4B as described for Fig. 1B, except that the binding reaction mixtures contained 0 mM, 2 mM, 5 mM, or 10 mM soluble 7-methyl GTP. Bound proteins were eluted by boiling in SDS sample buffer and analyzed by SDS-PAGE and autoradiography. The relative amounts of Vhs that bound the resin in the presence of various concentrations of 7-methyl GTP were quantified using a Storm Model 840 PhosphorImager (Molecular Dynamics, Inc.) and are plotted in the figure.
